Acid Vs Base Vs Alkaline . Acids, bases and alkalis are found in the laboratory and at home. November 13, 2019 at 9:32 am. Bases reduce the hydrogen ion concentration in water whereas acids increase them. She’s referring to the sodium hydroxide used to make soap; Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. Base is a chemical compound that reacts with acids to produce salts. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7, signifying a lower. Acids and bases neutralize each other. However, there is a slight difference. Acids and bases can neutralise each other. If a chemist tells you soapy water is basic, she isn’t calling it simple.
from www.mindbodygreen.com
However, there is a slight difference. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7, signifying a lower. If a chemist tells you soapy water is basic, she isn’t calling it simple. She’s referring to the sodium hydroxide used to make soap; Acids and bases can neutralise each other. Acids and bases neutralize each other. November 13, 2019 at 9:32 am. Bases reduce the hydrogen ion concentration in water whereas acids increase them. Base is a chemical compound that reacts with acids to produce salts.
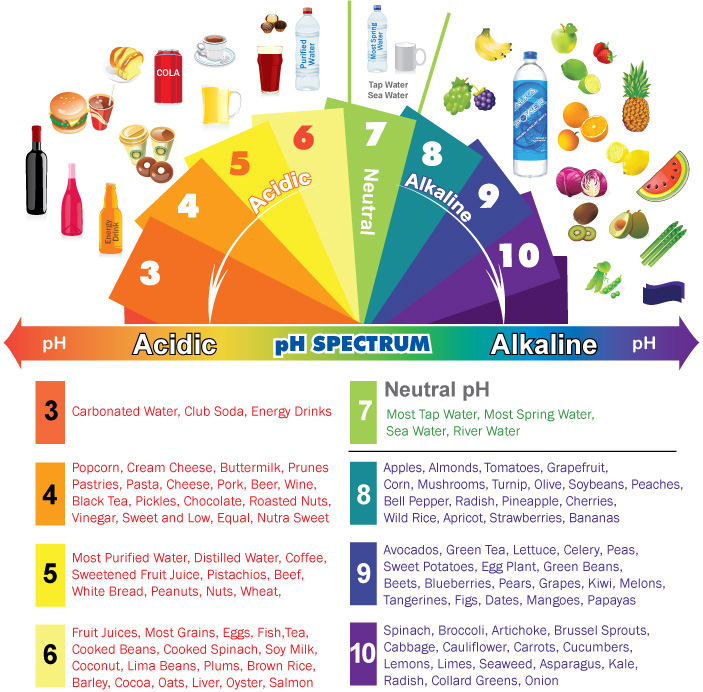
Alkaline & Acidic Foods Chart The pH Spectrum
Acid Vs Base Vs Alkaline She’s referring to the sodium hydroxide used to make soap; However, there is a slight difference. November 13, 2019 at 9:32 am. Base is a chemical compound that reacts with acids to produce salts. Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7, signifying a lower. Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. If a chemist tells you soapy water is basic, she isn’t calling it simple. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. She’s referring to the sodium hydroxide used to make soap; Acids, bases and alkalis are found in the laboratory and at home. Acids and bases neutralize each other. Bases reduce the hydrogen ion concentration in water whereas acids increase them. Acids and bases can neutralise each other.
From www.passhealthfoods.com
Acid vs. Alkaline Foods and the Benefits of an Alkaline Diet — Pass Acid Vs Base Vs Alkaline If a chemist tells you soapy water is basic, she isn’t calling it simple. She’s referring to the sodium hydroxide used to make soap; Acids, bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other. Bases reduce the hydrogen ion concentration in water whereas acids increase them. Acids have a ph value. Acid Vs Base Vs Alkaline.
From dashamlav.com
Acid vs Base Table of Differences between Acid and Base Acid Vs Base Vs Alkaline Acids and bases can neutralise each other. If a chemist tells you soapy water is basic, she isn’t calling it simple. Bases reduce the hydrogen ion concentration in water whereas acids increase them. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. Acids and bases neutralize each other. Acids, bases and alkalis. Acid Vs Base Vs Alkaline.
From sciencenotes.org
Alkaline vs Acidic Acid Vs Base Vs Alkaline Bases reduce the hydrogen ion concentration in water whereas acids increase them. Base is a chemical compound that reacts with acids to produce salts. However, there is a slight difference. November 13, 2019 at 9:32 am. She’s referring to the sodium hydroxide used to make soap; Acids and bases neutralize each other. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid. Acid Vs Base Vs Alkaline.
From 7esl.com
Acid vs. Base Difference Between Acid and Base • 7ESL Acid Vs Base Vs Alkaline Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. She’s referring to the sodium hydroxide used to make soap; Base is a chemical compound that reacts with acids to produce salts. Acids and bases can neutralise each other. Acids and bases neutralize each other. However, there is a slight difference. Alkaline and base are often used. Acid Vs Base Vs Alkaline.
From liveenergized.com
Alkaline Fruits Guide (Which Fruits Are Alkaline vs Acidic and Why) Acid Vs Base Vs Alkaline Acids and bases neutralize each other. November 13, 2019 at 9:32 am. Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7, signifying a lower. Acids and bases can neutralise each other. Bases reduce the hydrogen ion concentration in water whereas acids increase them. Alkaline. Acid Vs Base Vs Alkaline.
From useful-info24.blogspot.com
Acid/Alkaline Chart More of the right and less of the left. Useful Acid Vs Base Vs Alkaline If a chemist tells you soapy water is basic, she isn’t calling it simple. Bases reduce the hydrogen ion concentration in water whereas acids increase them. November 13, 2019 at 9:32 am. Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7, signifying a lower.. Acid Vs Base Vs Alkaline.
From www.mindbodygreen.com
Alkaline & Acidic Foods Chart The pH Spectrum Acid Vs Base Vs Alkaline Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. November 13, 2019 at 9:32 am. Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. Acids, bases and alkalis are found in the laboratory and at home. If a chemist tells you soapy water is basic, she isn’t calling. Acid Vs Base Vs Alkaline.
From www.vrogue.co
Ph Food Chart Acid Alkaline Food Chart 52 Off Elevate vrogue.co Acid Vs Base Vs Alkaline However, there is a slight difference. Bases reduce the hydrogen ion concentration in water whereas acids increase them. If a chemist tells you soapy water is basic, she isn’t calling it simple. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. Alkaline and base are often used interchangeably to describe substances with. Acid Vs Base Vs Alkaline.
From byjus.com
Difference between Acid and Base Differences btw Acid & Base in Acid Vs Base Vs Alkaline Acids, bases and alkalis are found in the laboratory and at home. Acids and bases neutralize each other. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. If a chemist tells you soapy water is basic, she isn’t calling it simple. Acids and bases can neutralise each other. She’s referring to the. Acid Vs Base Vs Alkaline.
From lagccnsdoer.commons.gc.cuny.edu
SCB 115 Lab 2 Exercise 4 pH, Acids, Bases, and Buffers Natural Acid Vs Base Vs Alkaline Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7, signifying a lower. She’s referring to the sodium hydroxide used to make soap; Alkali is a basic soluble. Acid Vs Base Vs Alkaline.
From pediaa.com
Difference Between Acid and Alkaline Definition, Properties, Examples Acid Vs Base Vs Alkaline Base is a chemical compound that reacts with acids to produce salts. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. November 13, 2019 at 9:32 am. If a chemist tells you soapy water is basic, she isn’t calling it simple. Acids and bases neutralize each other. However, there is a slight. Acid Vs Base Vs Alkaline.
From themasterchemistry.com
8 Differences Between Alkali And Basealkali Vs Base Acid Vs Base Vs Alkaline For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. If a chemist tells you soapy water is basic, she isn’t calling it simple. However, there is a slight difference. She’s referring to the sodium hydroxide used to make soap;. Acid Vs Base Vs Alkaline.
From www.vectorstock.com
Chart ph alkaline and acidic scale Royalty Free Vector Image Acid Vs Base Vs Alkaline If a chemist tells you soapy water is basic, she isn’t calling it simple. Acids, bases and alkalis are found in the laboratory and at home. Base is a chemical compound that reacts with acids to produce salts. She’s referring to the sodium hydroxide used to make soap; Acids and bases neutralize each other. Acids and bases can neutralise each. Acid Vs Base Vs Alkaline.
From pediaa.com
Difference Between Acid and Base Acid Vs Base Vs Alkaline Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. Acids, bases and alkalis are found in the laboratory and at home. If a chemist tells you soapy water is basic, she isn’t calling it simple. Base is a chemical compound that reacts with acids to produce salts. Bases reduce the hydrogen ion concentration in water whereas. Acid Vs Base Vs Alkaline.
From www.pickcomfort.com
The Best 6 Alkaline Water Pitcher Reviews 2021 Pick Comfort Acid Vs Base Vs Alkaline Acids, bases and alkalis are found in the laboratory and at home. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. She’s referring to the sodium hydroxide used to make soap; If a chemist tells. Acid Vs Base Vs Alkaline.
From www.sciencelearn.org.nz
pH scale — Science Learning Hub Acid Vs Base Vs Alkaline November 13, 2019 at 9:32 am. Bases reduce the hydrogen ion concentration in water whereas acids increase them. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7,. Acid Vs Base Vs Alkaline.
From www.pinterest.it
diagram of acids and bases Google Search Acids bases and salts Acid Vs Base Vs Alkaline Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. Acids have a ph value below 7, indicating a high concentration of hydrogen. Acid Vs Base Vs Alkaline.
From www.slideserve.com
PPT Acids and alkalis PowerPoint Presentation, free download ID142450 Acid Vs Base Vs Alkaline If a chemist tells you soapy water is basic, she isn’t calling it simple. However, there is a slight difference. Acids and bases can neutralise each other. She’s referring to the sodium hydroxide used to make soap; Acids, bases and alkalis are found in the laboratory and at home. Acids and bases neutralize each other. Alkaline and base are often. Acid Vs Base Vs Alkaline.
From www.phreshproducts.com
Alkaline vs Acidic Why You Should Care Phresh Products Acid Vs Base Vs Alkaline Bases reduce the hydrogen ion concentration in water whereas acids increase them. She’s referring to the sodium hydroxide used to make soap; Base is a chemical compound that reacts with acids to produce salts. Acids, bases and alkalis are found in the laboratory and at home. Acids and bases neutralize each other. November 13, 2019 at 9:32 am. However, there. Acid Vs Base Vs Alkaline.
From technotes.alconox.com
What to choose acid vs alkaline cleaner? TechNotes Critical Acid Vs Base Vs Alkaline If a chemist tells you soapy water is basic, she isn’t calling it simple. Acids and bases neutralize each other. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. She’s referring to the sodium hydroxide used to make soap; Acids, bases and alkalis are found in the laboratory and at home. For. Acid Vs Base Vs Alkaline.
From www.homemadetools.net
Acid and base guide infographic Acid Vs Base Vs Alkaline Acids, bases and alkalis are found in the laboratory and at home. Acids and bases neutralize each other. If a chemist tells you soapy water is basic, she isn’t calling it simple. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms. Acid Vs Base Vs Alkaline.
From www.worksheetsplanet.com
Acid and Bases Differences Acid Vs Base Vs Alkaline If a chemist tells you soapy water is basic, she isn’t calling it simple. Acids, bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other. Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. Base is a chemical compound that reacts with acids to produce salts. She’s referring. Acid Vs Base Vs Alkaline.
From blog.citysportsfitness.com
pH Wars Acidic vs. Alkaline Beverages City Sports Club Acid Vs Base Vs Alkaline Acids and bases neutralize each other. Base is a chemical compound that reacts with acids to produce salts. If a chemist tells you soapy water is basic, she isn’t calling it simple. She’s referring to the sodium hydroxide used to make soap; Bases reduce the hydrogen ion concentration in water whereas acids increase them. November 13, 2019 at 9:32 am.. Acid Vs Base Vs Alkaline.
From www.alamy.com
Diagram ph scale examples acidic hires stock photography and images Acid Vs Base Vs Alkaline Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. November 13, 2019 at 9:32 am. Acids and bases can neutralise each other. Bases reduce the hydrogen ion concentration in water whereas acids increase them. Acids and bases neutralize each other. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\). Acid Vs Base Vs Alkaline.
From morioh.com
Acids and Bases Basic Introduction Chemistry Acid Vs Base Vs Alkaline She’s referring to the sodium hydroxide used to make soap; Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. November 13, 2019 at 9:32 am. Bases reduce the hydrogen ion concentration in water whereas acids increase them. Acids and bases neutralize each other. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when. Acid Vs Base Vs Alkaline.
From dynamixinc.com
PH Alkalinity Mixing With Reaction Times Dynamix Agitators Acid Vs Base Vs Alkaline Base is a chemical compound that reacts with acids to produce salts. If a chemist tells you soapy water is basic, she isn’t calling it simple. Acids and bases neutralize each other. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. Bases reduce the hydrogen ion concentration in water whereas acids increase. Acid Vs Base Vs Alkaline.
From mungfali.com
Acid Base Neutral Chart Acid Vs Base Vs Alkaline Acids and bases neutralize each other. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. However, there is a slight difference. Acids and bases can neutralise each other. Bases reduce the hydrogen ion concentration in water whereas acids increase them. She’s referring to the sodium hydroxide used to make soap; November 13,. Acid Vs Base Vs Alkaline.
From www.kitchensanity.com
Alkaline Acid Food Chart & pH Foods Lists KitchenSanity Acid Vs Base Vs Alkaline Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. November 13, 2019 at 9:32 am. Acids, bases and alkalis are found in the laboratory and at home. She’s referring to the sodium hydroxide used to make soap; Acids and bases can neutralise each other. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid. Acid Vs Base Vs Alkaline.
From keithbrown.com
Balancing Acid vs. Alkaline Foods in Your Diet Keith Brown Acid Vs Base Vs Alkaline Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. Acids and bases neutralize each other. Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7, signifying a lower. Acids and bases can neutralise each other. If a. Acid Vs Base Vs Alkaline.
From www.healthymamamagazine.com
Reclaim Your Energy with Alkaline Foods Healthy Mama Mag Acid Vs Base Vs Alkaline For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7, signifying a lower. November 13, 2019 at 9:32 am. Acids, bases and alkalis are found in the laboratory. Acid Vs Base Vs Alkaline.
From examples.yourdictionary.com
Difference Between Acids and Bases Key Properties Acid Vs Base Vs Alkaline She’s referring to the sodium hydroxide used to make soap; Bases reduce the hydrogen ion concentration in water whereas acids increase them. If a chemist tells you soapy water is basic, she isn’t calling it simple. Acids, bases and alkalis are found in the laboratory and at home. Alkali is a basic soluble hydroxide of alkali metals or alkaline earth. Acid Vs Base Vs Alkaline.
From sciencenotes.org
Facts About Acids and Bases Acid Vs Base Vs Alkaline Acids and bases can neutralise each other. Acids, bases and alkalis are found in the laboratory and at home. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. November 13, 2019 at 9:32 am. Alkaline and base are often. Acid Vs Base Vs Alkaline.
From onlychemistrydiscussion.blogspot.com
Malik Xufyan Only Chemistry Discussion Base Vs Alkali Acid Vs Base Vs Alkaline Acids have a ph value below 7, indicating a high concentration of hydrogen ions (h⁺), while alkaline substances, or bases, have a ph above 7, signifying a lower. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. Acids and bases can neutralise each other. Bases reduce the hydrogen ion concentration in water. Acid Vs Base Vs Alkaline.
From www.clinicaleducation.org
Acid Or Alkali What Does Food Choice Have To Do With It? Clinical Acid Vs Base Vs Alkaline For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it dissolves in water. Acids, bases and alkalis are found in the laboratory and at home. Alkaline and base are often used interchangeably to describe substances with a ph level greater than 7. She’s referring to the sodium hydroxide used to make soap; Bases reduce the hydrogen. Acid Vs Base Vs Alkaline.
From arielle-well-wolfe.blogspot.com
Chemistry Form 4 Chapter 7 Acid and Base Acid Vs Base Vs Alkaline Acids, bases and alkalis are found in the laboratory and at home. Acids and bases neutralize each other. She’s referring to the sodium hydroxide used to make soap; Alkali is a basic soluble hydroxide of alkali metals or alkaline earth metals. November 13, 2019 at 9:32 am. Alkaline and base are often used interchangeably to describe substances with a ph. Acid Vs Base Vs Alkaline.