Copper Electrons Energy Level . 116 rows electrons orbit the atom's nucleus in energy levels. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. The valence band is the energy level in an atom that holds the outermost electrons. Copper ion (cu +, cu 2+) electron configuration. This table shows the pattern in the periodic table that mendeleev developed and. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds.
from www.nagwa.com
3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. Copper ion (cu +, cu 2+) electron configuration. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev developed and. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. The valence band is the energy level in an atom that holds the outermost electrons.
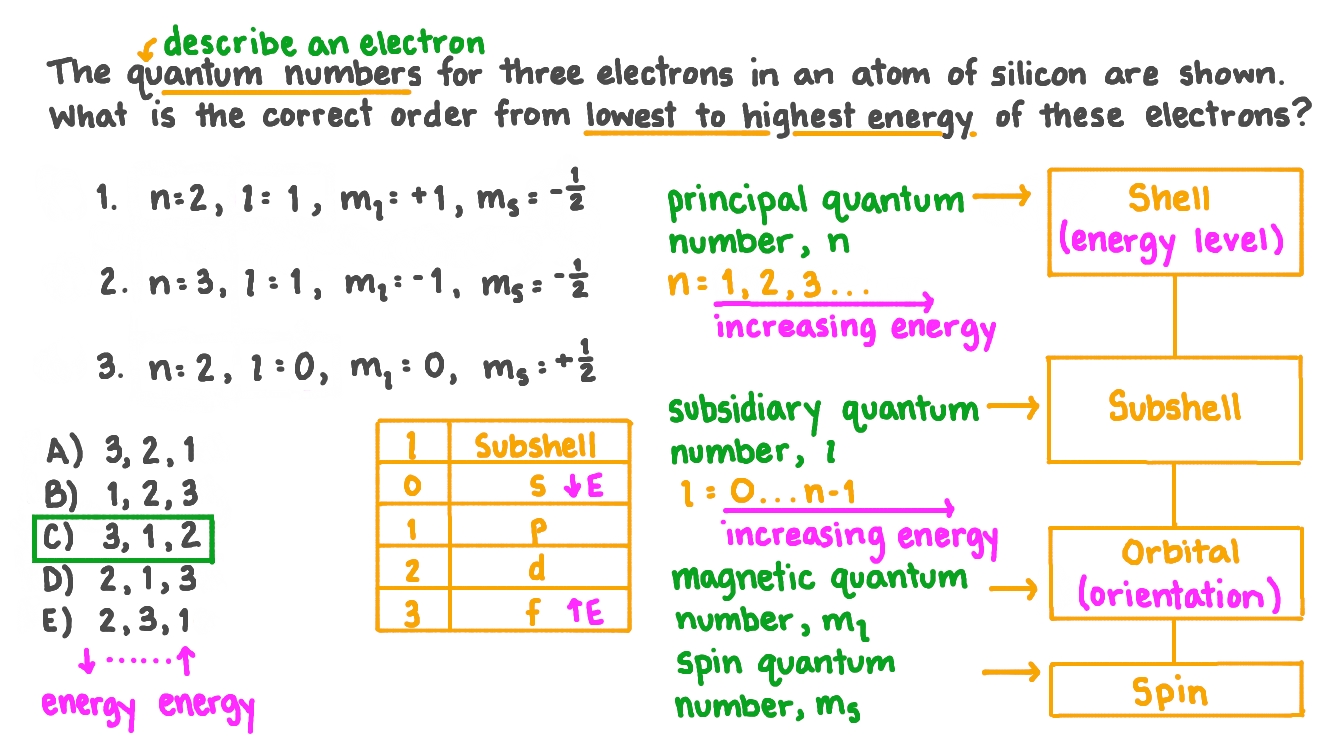
Question Video Ranking Three Electrons from Lowest to Highest Energy
Copper Electrons Energy Level These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. Copper ion (cu +, cu 2+) electron configuration. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev developed and. The valence band is the energy level in an atom that holds the outermost electrons. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons.
From dokumen.tips
(PPT) Electrons in Atoms. Energy level (shell) SublevelsOrbitalsNumber Copper Electrons Energy Level The valence band is the energy level in an atom that holds the outermost electrons. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d. Copper Electrons Energy Level.
From www.sliderbase.com
Energy Levels, Sublevels, Electrons Copper Electrons Energy Level Copper ion (cu +, cu 2+) electron configuration. This table shows the pattern in the periodic table that mendeleev developed and. 116 rows electrons orbit the atom's nucleus in energy levels. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. These electrons are important because they form bonds between atoms, such as. Copper Electrons Energy Level.
From wiringfixunripping.z21.web.core.windows.net
Valence Electrons Orbital Diagram Copper Electrons Energy Level This table shows the pattern in the periodic table that mendeleev developed and. Copper ion (cu +, cu 2+) electron configuration. The valence band is the energy level in an atom that holds the outermost electrons. 116 rows electrons orbit the atom's nucleus in energy levels. These electrons are important because they form bonds between atoms, such as covalent, ionic,. Copper Electrons Energy Level.
From proper-cooking.info
Copper Electron Dot Diagram Copper Electrons Energy Level At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. The ground state electron configuration of copper is. Copper Electrons Energy Level.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free download ID Copper Electrons Energy Level These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. This table shows the pattern in the periodic. Copper Electrons Energy Level.
From stock.adobe.com
Periodic Table of the Elements, Shell Structure of Copper Cu Copper Electrons Energy Level Copper ion (cu +, cu 2+) electron configuration. This table shows the pattern in the periodic table that mendeleev developed and. 116 rows electrons orbit the atom's nucleus in energy levels. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9. Copper Electrons Energy Level.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Copper Electrons Energy Level At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. This table shows the pattern in the periodic table that mendeleev developed and. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. These electrons are important because they form bonds between atoms, such as. Copper Electrons Energy Level.
From www.globetrotterscience.com
Unit 8 Atomic Structure Copper Electrons Energy Level Copper ion (cu +, cu 2+) electron configuration. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. The valence band is the energy level in an atom that holds the outermost electrons. 116 rows. Copper Electrons Energy Level.
From www.researchgate.net
Energylevel diagram of Cu with the observed absorption paths indicated Copper Electrons Energy Level The valence band is the energy level in an atom that holds the outermost electrons. Copper ion (cu +, cu 2+) electron configuration. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. This table shows the pattern in the periodic table that mendeleev developed and. These electrons are important because they form. Copper Electrons Energy Level.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper Electrons Energy Level The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. The valence band is the energy level in an atom that holds the outermost electrons. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. These electrons are important because they form bonds between atoms,. Copper Electrons Energy Level.
From www.slideserve.com
PPT Chapter 5 Electrons In Atoms PowerPoint Presentation, free Copper Electrons Energy Level This table shows the pattern in the periodic table that mendeleev developed and. The valence band is the energy level in an atom that holds the outermost electrons. 116 rows electrons orbit the atom's nucleus in energy levels. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. At absolute zero temperature, the. Copper Electrons Energy Level.
From stock.adobe.com
chart of electron configuration with each energy level for element in Copper Electrons Energy Level At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. Copper ion (cu +, cu 2+) electron configuration. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds.. Copper Electrons Energy Level.
From www.researchgate.net
Diagrams of the silicon and copper atoms. Download Scientific Diagram Copper Electrons Energy Level 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev developed and. At absolute zero. Copper Electrons Energy Level.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Copper Electrons Energy Level This table shows the pattern in the periodic table that mendeleev developed and. Copper ion (cu +, cu 2+) electron configuration. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. 116 rows electrons orbit the atom's nucleus in energy levels. The ground state electron configuration of copper is 1s 2 2s 2. Copper Electrons Energy Level.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper Electrons Energy Level At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. This table shows the pattern in the periodic table that mendeleev developed and. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. 116 rows electrons orbit the atom's nucleus in energy levels. The ground. Copper Electrons Energy Level.
From www.globalsino.com
Characteristic XRays and Their Peaks Copper Electrons Energy Level This table shows the pattern in the periodic table that mendeleev developed and. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. At absolute zero temperature, the valence band is the highest energy level that. Copper Electrons Energy Level.
From awesomehome.co
Copper Periodic Table Protons Neutrons And Electrons Awesome Home Copper Electrons Energy Level These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. 116 rows electrons orbit the atom's nucleus in energy levels. Copper ion (cu +, cu 2+) electron configuration. At absolute zero temperature, the valence band is. Copper Electrons Energy Level.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Image License Carlson Stock Art Copper Electrons Energy Level Copper ion (cu +, cu 2+) electron configuration. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. 116 rows electrons orbit the atom's nucleus in energy levels. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. The valence band is the energy level in. Copper Electrons Energy Level.
From www.expii.com
Valence Electrons — Definition & Importance Expii Copper Electrons Energy Level Copper ion (cu +, cu 2+) electron configuration. 116 rows electrons orbit the atom's nucleus in energy levels. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. The ground state electron configuration of copper. Copper Electrons Energy Level.
From www.researchgate.net
Ellingham diagram of copper oxides for an O 2 molecule. Energy level Copper Electrons Energy Level This table shows the pattern in the periodic table that mendeleev developed and. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. The valence band is the energy level in an atom that holds the outermost electrons. At absolute zero temperature, the valence band is the highest energy level that is completely. Copper Electrons Energy Level.
From socratic.org
Do electrons fill the lower energy levels first? Socratic Copper Electrons Energy Level This table shows the pattern in the periodic table that mendeleev developed and. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. These electrons are important because they form bonds between atoms, such as covalent,. Copper Electrons Energy Level.
From quizlet.com
Diagram and label electron energy levels in Bohr's model. Quizlet Copper Electrons Energy Level The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. This table shows the pattern in the periodic table that mendeleev developed and. The valence band is the energy level in an atom that holds the outermost electrons. At absolute zero temperature, the valence band is the highest energy level that is completely. Copper Electrons Energy Level.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.2.4 Electronic Configurations翰林国际教育 Copper Electrons Energy Level 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. This table shows the pattern in the periodic table that mendeleev developed and. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. At absolute zero temperature, the valence band is the highest energy level that. Copper Electrons Energy Level.
From www.alamy.com
Symbol and electron diagram for Copper illustration Stock Vector Image Copper Electrons Energy Level 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev developed and. Copper ion (cu +, cu 2+) electron configuration. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. The valence band is the energy level in an atom that holds. Copper Electrons Energy Level.
From stock.adobe.com
Cu Copper Element Information Facts, Properties, Trends, Uses and Copper Electrons Energy Level Copper ion (cu +, cu 2+) electron configuration. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. The valence band is the energy level in an atom that holds the outermost electrons. This table shows the pattern in the periodic table that mendeleev developed and. 116 rows electrons orbit the atom's. Copper Electrons Energy Level.
From opentextbc.ca
Organization of Electrons in Atoms Introductory Chemistry 1st Copper Electrons Energy Level 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. Copper ion (cu +, cu 2+) electron configuration. The valence band is the energy level in an atom that holds the outermost electrons. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. The ground. Copper Electrons Energy Level.
From www.nagwa.com
Question Video Ranking Three Electrons from Lowest to Highest Energy Copper Electrons Energy Level The valence band is the energy level in an atom that holds the outermost electrons. Copper ion (cu +, cu 2+) electron configuration. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. These electrons. Copper Electrons Energy Level.
From sciencing.com
Energy Level Definition, Equation (w/ Diagrams) Sciencing Copper Electrons Energy Level 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev developed and. At absolute zero. Copper Electrons Energy Level.
From www.researchgate.net
Energy level diagram of 3d electrons in Cu 3+ cation. Download Copper Electrons Energy Level The valence band is the energy level in an atom that holds the outermost electrons. 116 rows electrons orbit the atom's nucleus in energy levels. Copper ion (cu +, cu 2+) electron configuration. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. The ground state electron configuration of copper is 1s 2. Copper Electrons Energy Level.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electrons Energy Level The valence band is the energy level in an atom that holds the outermost electrons. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev developed and. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. The ground state electron. Copper Electrons Energy Level.
From www.chegg.com
Solved 1. (20 points) The Fermi energy in copper is 7.04 eV. Copper Electrons Energy Level These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. This table shows the pattern in the periodic table that mendeleev developed and. At absolute zero temperature, the valence band is the highest energy level that. Copper Electrons Energy Level.
From lefteris-kaliambos.fandom.com
EXPLANATION OF IONIZATIONS Lefteris Kaliambos Wiki Fandom Copper Electrons Energy Level These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. Copper ion (cu +, cu 2+) electron configuration. This table shows the pattern in the periodic table that mendeleev developed and. At absolute zero temperature, the. Copper Electrons Energy Level.
From www.dreamstime.com
Copper stock illustration. Illustration of chemistry 89682579 Copper Electrons Energy Level These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. The valence band is the energy level in an atom that holds the outermost electrons. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. The ground state electron configuration of copper is 1s 2. Copper Electrons Energy Level.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Copper Electrons Energy Level 116 rows electrons orbit the atom's nucleus in energy levels. At absolute zero temperature, the valence band is the highest energy level that is completely filled with electrons. This table shows the pattern in the periodic table that mendeleev developed and. 3d 1 0 (1 s)4s 2 s 1/2 0.000 sm90 3d 9 4s 2 2 d 5/2. Copper ion. Copper Electrons Energy Level.
From www.researchgate.net
Energy level diagram showing electron transitions producing Fe K and L Copper Electrons Energy Level 116 rows electrons orbit the atom's nucleus in energy levels. These electrons are important because they form bonds between atoms, such as covalent, ionic, or metallic bonds. Copper ion (cu +, cu 2+) electron configuration. The valence band is the energy level in an atom that holds the outermost electrons. At absolute zero temperature, the valence band is the highest. Copper Electrons Energy Level.