Explain Inductive Effect And Resonance . While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. The inductive effect is addictive; Substituents determine the reaction direction by resonance or inductive effect. Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Let’s start today’s discussion with simple terms: More chlorine atoms have an overall stronger effect,. Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule.
from leah4sci.com
Let’s start today’s discussion with simple terms: While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. The inductive effect is addictive; More chlorine atoms have an overall stronger effect,. Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. Substituents determine the reaction direction by resonance or inductive effect. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical.
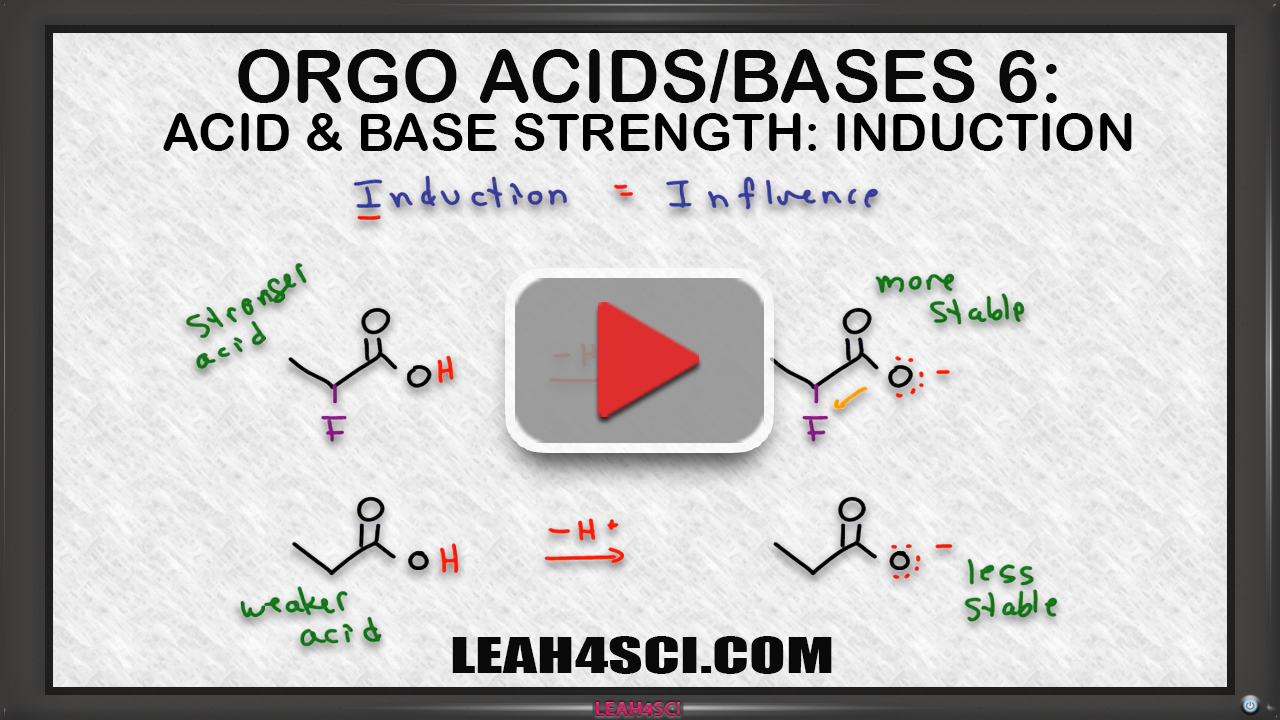
Inductive Effect on Acid Base Strength in Organic Chemistry
Explain Inductive Effect And Resonance The inductive effect is addictive; The inductive effect is addictive; Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. More chlorine atoms have an overall stronger effect,. Substituents determine the reaction direction by resonance or inductive effect. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Let’s start today’s discussion with simple terms: Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and.
From askfilo.com
Distinguish A. Inductive effect and resonance effe Filo Explain Inductive Effect And Resonance The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. More chlorine atoms have an overall stronger effect,. The inductive effect is addictive; Let’s start today’s discussion with simple terms: The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. Both inductive and mesomeric (resonance) effects tell us. Explain Inductive Effect And Resonance.
From brainly.in
5 difference between resonance effect and inductive effect Brainly.in Explain Inductive Effect And Resonance The inductive effect is addictive; Let’s start today’s discussion with simple terms: The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. More chlorine atoms have an overall stronger effect,. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. Substituents determine the reaction direction by resonance or. Explain Inductive Effect And Resonance.
From www.pdfprof.com
PDF inductive effect and resonance effect PDF Télécharger Download Explain Inductive Effect And Resonance More chlorine atoms have an overall stronger effect,. Let’s start today’s discussion with simple terms: Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. The key difference between inductive and resonance effects. Explain Inductive Effect And Resonance.
From byjus.com
What is the difference between the inductive effect and the resonance Explain Inductive Effect And Resonance The inductive effect is addictive; Let’s start today’s discussion with simple terms: While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. More chlorine atoms have an overall stronger effect,. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Substituents determine the reaction. Explain Inductive Effect And Resonance.
From www.pinterest.com
Difference Between Inductive and Resonance Effect Teaching chemistry Explain Inductive Effect And Resonance Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. More chlorine atoms have an overall stronger effect,. Let’s start today’s discussion with simple terms: The key difference between inductive and resonance effects is that inductive effects are caused by polarization. Explain Inductive Effect And Resonance.
From www.youtube.com
Inductive effect and resonance effect l part2 l class 11l Organic Explain Inductive Effect And Resonance The inductive effect is addictive; The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. Let’s start today’s discussion with simple terms: While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Substituents determine the reaction direction by resonance or. Explain Inductive Effect And Resonance.
From www.learnpick.in
Inductive Resonance And Hyper Conjugation Effects PowerPoint Slides Explain Inductive Effect And Resonance The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. The inductive effect is addictive;. Explain Inductive Effect And Resonance.
From www.youtube.com
Comparing Acidities Inductive Effects YouTube Explain Inductive Effect And Resonance While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Substituents determine the reaction direction by resonance or inductive effect. Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. The inductive effect is addictive; The key difference between inductive and resonance effects is. Explain Inductive Effect And Resonance.
From www.vrogue.co
What Is The Difference Between Inductive And vrogue.co Explain Inductive Effect And Resonance More chlorine atoms have an overall stronger effect,. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. Substituents determine. Explain Inductive Effect And Resonance.
From www.vrogue.co
What Is The Difference Between Inductive And vrogue.co Explain Inductive Effect And Resonance The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. The key difference between inductive and resonance effects is that. Explain Inductive Effect And Resonance.
From www.meritnation.com
what is the difference between inductive and electromeric effect Explain Inductive Effect And Resonance The inductive effect is addictive; Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. Let’s start today’s discussion with simple terms: Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The key difference between inductive. Explain Inductive Effect And Resonance.
From www.vrogue.co
Inductive Effect Electromeric Effect Resonance Effect vrogue.co Explain Inductive Effect And Resonance While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. The inductive effect is addictive; Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. More chlorine atoms have an. Explain Inductive Effect And Resonance.
From www.chemistrysteps.com
Inductive and Resonance (Mesomeric) Effects Chemistry Steps Explain Inductive Effect And Resonance The inductive effect is addictive; Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. More chlorine atoms have an overall stronger effect,. Substituents determine the reaction direction by resonance or inductive effect. Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. While both the inductive effect and the resonance. Explain Inductive Effect And Resonance.
From www.youtube.com
Inductive Effect Reaction Mechanisms YouTube Explain Inductive Effect And Resonance The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. The inductive effect is addictive; Let’s start today’s discussion with simple terms: Resonance effect. Explain Inductive Effect And Resonance.
From www.reddit.com
Inductive effect, Mesomeric effect, Resonance Explain Inductive Effect And Resonance Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The inductive effect is addictive; Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. Substituents determine the reaction direction by resonance or inductive effect. More chlorine. Explain Inductive Effect And Resonance.
From www.differencebetween.com
Difference Between Hyperconjugation and Inductive Effect Compare the Explain Inductive Effect And Resonance While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Let’s start today’s discussion with simple terms: More chlorine atoms have an overall stronger effect,. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. Both inductive and mesomeric (resonance). Explain Inductive Effect And Resonance.
From www.slideserve.com
PPT Aromatic Nitration Mechanism PowerPoint Presentation, free Explain Inductive Effect And Resonance Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. The inductive effect is addictive; More chlorine atoms have an overall stronger effect,. The inductive effect is the charge dispersal effect of electronegative. Explain Inductive Effect And Resonance.
From www.youtube.com
Induction vs Resonance (Rules of Organic Chemistry 4) YouTube Explain Inductive Effect And Resonance Substituents determine the reaction direction by resonance or inductive effect. Let’s start today’s discussion with simple terms: The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. The key difference between inductive and resonance effects is that inductive effects are caused. Explain Inductive Effect And Resonance.
From www.youtube.com
What is Inductive Effect ? YouTube Explain Inductive Effect And Resonance Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. The inductive effect is addictive; Let’s start today’s discussion with simple terms: The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. More chlorine atoms have an. Explain Inductive Effect And Resonance.
From studylib.net
Resonance and Inductive Effects Explain Inductive Effect And Resonance More chlorine atoms have an overall stronger effect,. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. Let’s start today’s discussion with simple terms: Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. Substituents determine the reaction direction by resonance or inductive effect. The inductive effect. Explain Inductive Effect And Resonance.
From www.difference.wiki
Inductive Effect vs. Resonance Effect What’s the Difference? Explain Inductive Effect And Resonance The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. The inductive effect is addictive; Substituents determine the reaction direction by resonance or inductive effect. More chlorine atoms have an overall stronger effect,. While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in. Explain Inductive Effect And Resonance.
From askfilo.com
What is Inductive effect? Resonance Hyperconjugation Filo Explain Inductive Effect And Resonance Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. More chlorine atoms have an overall stronger effect,. Substituents determine the reaction direction by resonance or inductive effect. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. The inductive effect is the charge dispersal effect of electronegative. Explain Inductive Effect And Resonance.
From scienceinfo.com
Inductive Effect Types, Uses, Stability Explain Inductive Effect And Resonance The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Substituents determine the reaction direction by resonance or inductive effect. Let’s start today’s discussion with simple terms: More chlorine atoms have an overall stronger effect,. Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. While both the inductive effect and the. Explain Inductive Effect And Resonance.
From www.studypool.com
SOLUTION Inductive Effect Resonance Effect & Hyperconjugation Explain Inductive Effect And Resonance More chlorine atoms have an overall stronger effect,. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The inductive effect is addictive; Let’s start today’s discussion with simple terms: While both the inductive effect and the resonance. Explain Inductive Effect And Resonance.
From www.studypool.com
SOLUTION Applications of inductive effect resonance effect Studypool Explain Inductive Effect And Resonance Let’s start today’s discussion with simple terms: The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. The inductive effect is addictive; The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. More chlorine atoms have an overall stronger effect,. Resonance effect is the conjugation between the ring. Explain Inductive Effect And Resonance.
From byjus.com
What is the difference between the inductive effect and the resonance Explain Inductive Effect And Resonance While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. More chlorine atoms have an overall stronger effect,. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Substituents determine the reaction direction by resonance or inductive effect. The inductive effect is addictive; Resonance. Explain Inductive Effect And Resonance.
From www.teachmint.com
Order Of Inductive Effect Chemistry Notes Teachmint Explain Inductive Effect And Resonance Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. The inductive effect is addictive; While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. More chlorine atoms have an overall stronger effect,. Substituents determine the reaction direction by resonance or inductive effect. The. Explain Inductive Effect And Resonance.
From www.vrogue.co
Inductive Effect Electromeric Effect Resonance Effect vrogue.co Explain Inductive Effect And Resonance The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Substituents determine the reaction direction by resonance or inductive effect. Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. Let’s start today’s discussion with simple terms: More chlorine atoms have an overall stronger effect,. Resonance effect is the conjugation between the. Explain Inductive Effect And Resonance.
From chemistnotes.com
Inductive Effect Definitions/ Examples Chemistry Notes Explain Inductive Effect And Resonance Substituents determine the reaction direction by resonance or inductive effect. More chlorine atoms have an overall stronger effect,. Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. The inductive effect is addictive; Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. The key difference between inductive and resonance effects. Explain Inductive Effect And Resonance.
From www.doubtnut.com
Give three points of difference between inductive effect and resonance Explain Inductive Effect And Resonance While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. Substituents determine the reaction direction by resonance or inductive effect. The inductive effect is the charge dispersal effect of electronegative. Explain Inductive Effect And Resonance.
From www.vrogue.co
Inductive Effect Electromeric Effect Resonance Effect vrogue.co Explain Inductive Effect And Resonance More chlorine atoms have an overall stronger effect,. Let’s start today’s discussion with simple terms: Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. The inductive effect is addictive; While both the inductive effect and the resonance effect influence the. Explain Inductive Effect And Resonance.
From leah4sci.com
Inductive Effect on Acid Base Strength in Organic Chemistry Explain Inductive Effect And Resonance Let’s start today’s discussion with simple terms: While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Substituents determine the reaction direction by resonance or inductive effect. More chlorine atoms have an overall stronger effect,. The inductive effect is addictive; Both inductive and mesomeric (resonance) effects tell us. Explain Inductive Effect And Resonance.
From www.youtube.com
Organic Chemistry Carbocation Stability, Inductive Effect, and Explain Inductive Effect And Resonance The key difference between inductive and resonance effects is that inductive effects are caused by polarization of chemical. The inductive effect is addictive; While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing.. Explain Inductive Effect And Resonance.
From www.chegg.com
Classify the following substituents according to Explain Inductive Effect And Resonance Substituents determine the reaction direction by resonance or inductive effect. More chlorine atoms have an overall stronger effect,. While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. Both inductive and mesomeric (resonance) effects tell us whether one part of the molecule. The inductive effect is the charge. Explain Inductive Effect And Resonance.
From pdfprof.com
explain inductive effect Explain Inductive Effect And Resonance More chlorine atoms have an overall stronger effect,. While both the inductive effect and the resonance effect influence the electron distribution in organic molecules, they differ in their mechanisms and. The inductive effect is the charge dispersal effect of electronegative atoms through σ bonds. Let’s start today’s discussion with simple terms: Both inductive and mesomeric (resonance) effects tell us whether. Explain Inductive Effect And Resonance.