Titration Short Definition . Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured.
from www.chemistryscl.com
titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by.
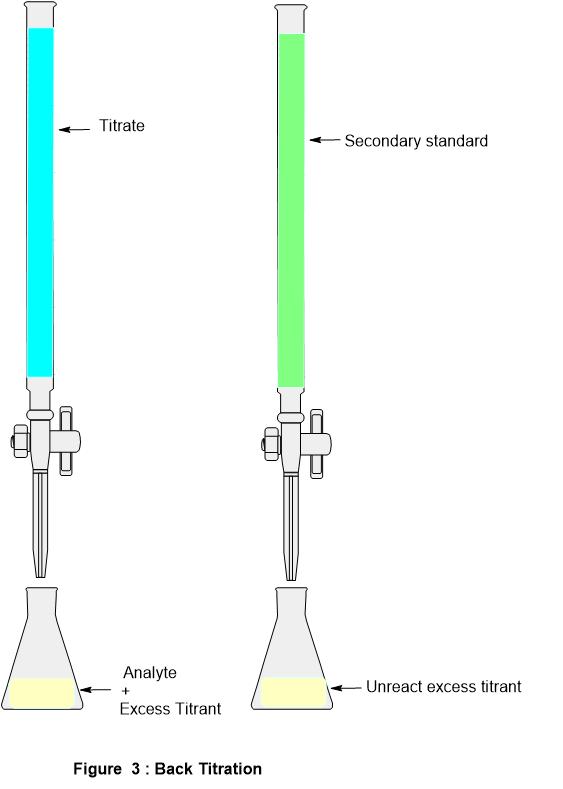
Titrimetry, Titration Classifications, Standard solutions, Equivalence
Titration Short Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Short Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration, process of chemical analysis in which the quantity. Titration Short Definition.
From www.youtube.com
titration part1 definition YouTube Titration Short Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution. Titration Short Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Short Definition a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another. Titration Short Definition.
From exovmncod.blob.core.windows.net
Indicator Definition In A Titration at Maria Little blog Titration Short Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution. Titration Short Definition.
From dxozdakva.blob.core.windows.net
Titrate Away Definition at Joe Ratliff blog Titration Short Definition titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is a quantitative analysis to determine the concentration. Titration Short Definition.
From www.numerade.com
SOLVED Define the following terms used in volumetric/titrimetric Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. Titration involves the gradual addition of a reagent of known concentration, known as. Titration Short Definition.
From dxompffry.blob.core.windows.net
Titration Formula Class 12 at Donald Rios blog Titration Short Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. a method or the activity of finding exactly how much of. Titration Short Definition.
From www.hoddereducationmagazines.com
Performing the perfect titration Hodder Education Magazines Titration Short Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a method or the activity of finding exactly how much of a. Titration Short Definition.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is the process in which one solution is added to another solution such that it. Titration Short Definition.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Titration involves the gradual addition of a reagent of. Titration Short Definition.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. a method or the activity of finding exactly. Titration Short Definition.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Short Definition titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is the process in which one solution is added to. Titration Short Definition.
From www.tes.com
KS3 GCSE Chemistry Introduction To Titrations Lesson Presentation and Titration Short Definition titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a method or the activity of finding exactly how much. Titration Short Definition.
From slideplayer.com
Acids and Bases. ppt download Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is a quantitative analysis to determine the. Titration Short Definition.
From www.youtube.com
A short guide to titration calculations YouTube Titration Short Definition titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is the process in which one solution is added to another solution such that. Titration Short Definition.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. Titration Short Definition.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Short Definition titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is a quantitative analysis to determine the. Titration Short Definition.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is a quantitative analysis to determine the. Titration Short Definition.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Short Definition titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. titration is the slow addition of one. Titration Short Definition.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Short Definition a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another. Titration Short Definition.
From www.docsity.com
Short Notes of Redox titration Cheat Sheet Chemistry Docsity Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a method or the activity of finding exactly how much of a. Titration Short Definition.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. titration is the process in which one solution is added. Titration Short Definition.
From chemistnotes.com
Redox Titration Definition, Requirements, Indicators, and 5 Reliable Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Titration Short Definition.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Titration Short Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution. Titration Short Definition.
From www.picxsexy.com
Solution Kimia Titrasi Asam Basa Acid Base Titration Chemistry Titration Short Definition a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the slow addition of one solution of a known concentration (called. Titration Short Definition.
From chemistnotes.com
Complexometric Titration Definition, Types, Indicators, and 5 Reliable Titration Short Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a method or the activity of finding exactly how much of a substance there is in. Titration Short Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is a quantitative analysis to determine the. Titration Short Definition.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Short Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is the slow addition of one solution of a known concentration (called. Titration Short Definition.
From chem-textbook.ucalgary.ca
Finding the Endpoint of a Titration UCalgary Chemistry Textbook Titration Short Definition a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is a quantitative analysis to determine the concentration of an unknown solution. Titration Short Definition.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog Titration Short Definition a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration, process of chemical analysis in which the quantity. Titration Short Definition.
From www.youtube.com
Viva voce Questions Part2 on Complexometric titrations/EDTA titration Titration Short Definition titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Titration involves the gradual addition of a reagent. Titration Short Definition.
From dxozdakva.blob.core.windows.net
Titrate Away Definition at Joe Ratliff blog Titration Short Definition titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is a quantitative analysis to determine the concentration. Titration Short Definition.
From www.thoughtco.com
Titration Definition (Chemistry) Titration Short Definition a method or the activity of finding exactly how much of a substance there is in a solution by gradually adding measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration involves the gradual addition of a reagent of known concentration, known as. Titration Short Definition.
From www.youtube.com
Chemistry & Biology Define the Term Titration YouTube Titration Short Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. titration is the process in which one solution is added. Titration Short Definition.
From www.mt.com
Get a Deeper Insight into the Basics of Titration Titration Short Definition titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be. titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at. titration, process of chemical analysis in which. Titration Short Definition.