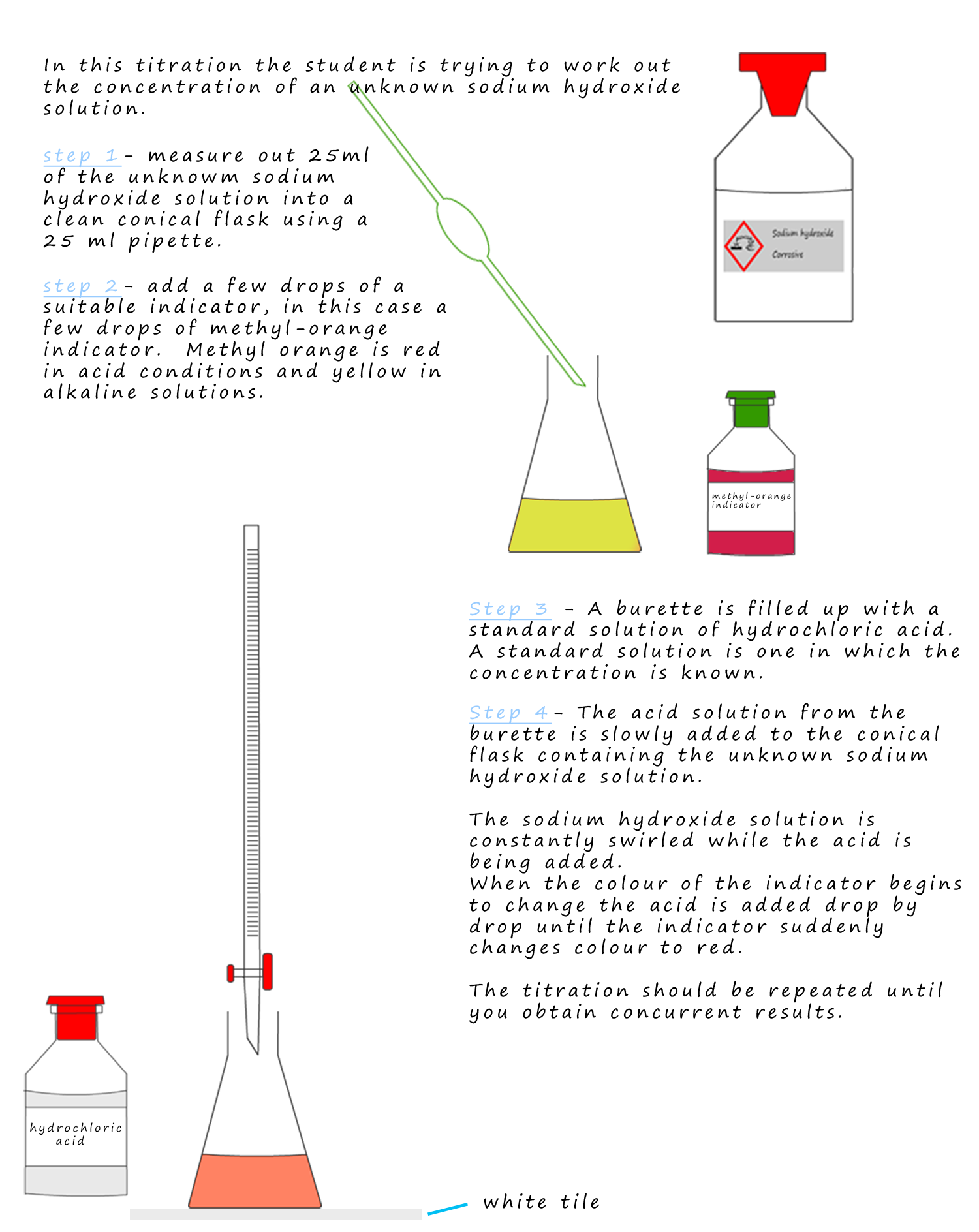
Why Is The First Titration Not Used . It's an analytical technique that can be used to find the concentration. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. titration is often used to determine the concentration of a solution. Titrations one and three have results that are within 0.20 cm 3 of each other but. anyone who's studied chemistry will be overly familiar with titrations. you can ignore the first (rough) titration result. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. this is the first titration, and it is not very precise. It should be excluded from any calculations. Perform 2 or 3 more titrations for.
from www.science-revision.co.uk
Perform 2 or 3 more titrations for. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. anyone who's studied chemistry will be overly familiar with titrations. It's an analytical technique that can be used to find the concentration. titration is often used to determine the concentration of a solution. Titrations one and three have results that are within 0.20 cm 3 of each other but. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. It should be excluded from any calculations. this is the first titration, and it is not very precise. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
Titrations
Why Is The First Titration Not Used anyone who's studied chemistry will be overly familiar with titrations. Titrations one and three have results that are within 0.20 cm 3 of each other but. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. you can ignore the first (rough) titration result. Perform 2 or 3 more titrations for. this is the first titration, and it is not very precise. It's an analytical technique that can be used to find the concentration. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is often used to determine the concentration of a solution. anyone who's studied chemistry will be overly familiar with titrations. It should be excluded from any calculations.
From www.youtube.com
Titration Calculations YouTube Why Is The First Titration Not Used titration is often used to determine the concentration of a solution. you can ignore the first (rough) titration result. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. this is the first titration, and it is not very precise. Perform 2 or 3. Why Is The First Titration Not Used.
From dxolbfltl.blob.core.windows.net
Halfway Point For Titration Curve at Mildred Cruz blog Why Is The First Titration Not Used Perform 2 or 3 more titrations for. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. It's an analytical technique that can be used to find the concentration. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and.. Why Is The First Titration Not Used.
From www.scribd.com
NonAqueous Acid Base Titration Acid Titration Why Is The First Titration Not Used Titrations one and three have results that are within 0.20 cm 3 of each other but. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Why Is The First Titration Not Used.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Why Is The First Titration Not Used Titrations one and three have results that are within 0.20 cm 3 of each other but. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is often used to determine the concentration of a solution. It's an analytical technique that can be used to find the. Why Is The First Titration Not Used.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog Why Is The First Titration Not Used titration is often used to determine the concentration of a solution. Titrations one and three have results that are within 0.20 cm 3 of each other but. this is the first titration, and it is not very precise. anyone who's studied chemistry will be overly familiar with titrations. you can ignore the first (rough) titration result.. Why Is The First Titration Not Used.
From mungfali.com
Acid Base Titration Calculation Why Is The First Titration Not Used you can ignore the first (rough) titration result. It's an analytical technique that can be used to find the concentration. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. Titrations one and three have results that are within 0.20 cm 3 of each other but. It should be excluded from. Why Is The First Titration Not Used.
From www.myxxgirl.com
What Is Double Titration Theory By Using Sulphuric Acid Chemistry My Why Is The First Titration Not Used the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. Perform 2 or 3 more titrations for. you can ignore the first (rough) titration result. this is the first titration, and it is not very precise. anyone who's studied chemistry will be overly familiar. Why Is The First Titration Not Used.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog Why Is The First Titration Not Used It should be excluded from any calculations. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. you can ignore the first (rough) titration result. titration is often used. Why Is The First Titration Not Used.
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman Why Is The First Titration Not Used Perform 2 or 3 more titrations for. It's an analytical technique that can be used to find the concentration. Titrations one and three have results that are within 0.20 cm 3 of each other but. you can ignore the first (rough) titration result. a titration is a laboratory technique used to precisely measure molar concentration of an unknown. Why Is The First Titration Not Used.
From www.hotzxgirl.com
Acid Base Titrations Titration Curve Hot Sex Picture Why Is The First Titration Not Used this is the first titration, and it is not very precise. Titrations one and three have results that are within 0.20 cm 3 of each other but. It should be excluded from any calculations. anyone who's studied chemistry will be overly familiar with titrations. It's an analytical technique that can be used to find the concentration. you. Why Is The First Titration Not Used.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Why Is The First Titration Not Used you can ignore the first (rough) titration result. Perform 2 or 3 more titrations for. anyone who's studied chemistry will be overly familiar with titrations. It's an analytical technique that can be used to find the concentration. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. a titration. Why Is The First Titration Not Used.
From socratic.org
How many moles of HX have been added at the equivalence point? Socratic Why Is The First Titration Not Used In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. Perform 2 or 3 more titrations for. It's an analytical technique that can be used to find the concentration. It should be excluded from any calculations. a titration is a laboratory technique used to precisely measure molar concentration of an unknown. Why Is The First Titration Not Used.
From www.vrogue.co
Solved Draw A Titration Curve For Ala Use Figure 3 10 vrogue.co Why Is The First Titration Not Used In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. It's an analytical technique that can be used to find the concentration. It should be excluded from any calculations. titration is often used to determine the concentration of a solution. Perform 2 or 3 more titrations for. Titrations one and three. Why Is The First Titration Not Used.
From hicensvanderkruijs.blogspot.com
The Graph Shows The Titration Curves Of A 1M Solution / Consider The Why Is The First Titration Not Used this is the first titration, and it is not very precise. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It's an analytical technique that can be used to find the concentration. the objective of the titration is to find the volume of the base (of. Why Is The First Titration Not Used.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Why Is The First Titration Not Used It's an analytical technique that can be used to find the concentration. It should be excluded from any calculations. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. anyone who's studied chemistry will be overly familiar with titrations. Titrations one and three have results that. Why Is The First Titration Not Used.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Why Is The First Titration Not Used a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. this is the first titration, and it is not very precise. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. Titrations one and three have results that are within. Why Is The First Titration Not Used.
From www.facebook.com
Why have drug overdose deaths suddenly fallen for the first time in a Why Is The First Titration Not Used Titrations one and three have results that are within 0.20 cm 3 of each other but. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It's an analytical technique that can be used to find the concentration. Perform 2 or 3 more titrations for. you can ignore. Why Is The First Titration Not Used.
From vanesfirstworldproblems.blogspot.com
Vane's First World Problems Titrations Why Is The First Titration Not Used It should be excluded from any calculations. It's an analytical technique that can be used to find the concentration. this is the first titration, and it is not very precise. you can ignore the first (rough) titration result. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Why Is The First Titration Not Used.
From dxobegonq.blob.core.windows.net
Titration Curve Labels at Ok Moore blog Why Is The First Titration Not Used It's an analytical technique that can be used to find the concentration. you can ignore the first (rough) titration result. anyone who's studied chemistry will be overly familiar with titrations. titration is often used to determine the concentration of a solution. this is the first titration, and it is not very precise. It should be excluded. Why Is The First Titration Not Used.
From dandkmotorsports.com
Tris Buffer Recipe Table Dandk Organizer Why Is The First Titration Not Used Perform 2 or 3 more titrations for. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It should be excluded from any calculations. this is the first titration, and. Why Is The First Titration Not Used.
From www.youtube.com
Performing Antibody Titration YouTube Why Is The First Titration Not Used anyone who's studied chemistry will be overly familiar with titrations. you can ignore the first (rough) titration result. Perform 2 or 3 more titrations for. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. this is the first titration, and it is not. Why Is The First Titration Not Used.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Why Is The First Titration Not Used Perform 2 or 3 more titrations for. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. It's an analytical technique that can be used to find the concentration. Titrations one and three have results that are within 0.20 cm 3 of each other but. the objective of the titration is. Why Is The First Titration Not Used.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Why Is The First Titration Not Used titration is often used to determine the concentration of a solution. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. anyone who's studied chemistry will be overly familiar with titrations. It should be excluded from any calculations. you can ignore the first (rough). Why Is The First Titration Not Used.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Why Is The First Titration Not Used In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. this is the first titration, and it is not very precise. Perform 2 or 3 more titrations for. you can ignore the first (rough) titration result. anyone who's studied chemistry will be overly familiar with titrations. titration is. Why Is The First Titration Not Used.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Why Is The First Titration Not Used the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. Perform 2 or 3 more titrations for. titration is often used to determine the concentration of a solution. you can ignore the first (rough) titration result. It's an analytical technique that can be used to. Why Is The First Titration Not Used.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Why Is The First Titration Not Used the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. anyone who's studied chemistry will be overly familiar with titrations. Titrations one and three have results that are within 0.20 cm 3 of each other but. a titration is a laboratory technique used to precisely. Why Is The First Titration Not Used.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Why Is The First Titration Not Used Perform 2 or 3 more titrations for. titration is often used to determine the concentration of a solution. It's an analytical technique that can be used to find the concentration. Titrations one and three have results that are within 0.20 cm 3 of each other but. It should be excluded from any calculations. a titration is a laboratory. Why Is The First Titration Not Used.
From www.vrogue.co
The Graphs Labeled A And B Show The Titration Curves vrogue.co Why Is The First Titration Not Used titration is often used to determine the concentration of a solution. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. this is the first titration, and it is not very precise. In many cases it is not a simple matter to obtain a pure. Why Is The First Titration Not Used.
From www.facebook.com
Why is Assessment for Learning important in Science lessons Why Is The First Titration Not Used Perform 2 or 3 more titrations for. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the objective of the titration is to find the volume of the base (of known concentration) necessary to neutralize the acid, and. Titrations one and three have results that are within. Why Is The First Titration Not Used.
From www.reagent.co.uk
Who Invented Titration? The Science Blog Why Is The First Titration Not Used Perform 2 or 3 more titrations for. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. you can ignore the first (rough) titration result. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It should be excluded from. Why Is The First Titration Not Used.
From studylib.net
2 POTENTIOMETRIC TITRATIONS Why Is The First Titration Not Used It should be excluded from any calculations. Perform 2 or 3 more titrations for. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titrations one and three have results that are within 0.20 cm 3 of each other but. anyone who's studied chemistry will be overly familiar. Why Is The First Titration Not Used.
From theedge.com.hk
Chemistry How To Titration The Edge Why Is The First Titration Not Used It's an analytical technique that can be used to find the concentration. Titrations one and three have results that are within 0.20 cm 3 of each other but. anyone who's studied chemistry will be overly familiar with titrations. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. It should be. Why Is The First Titration Not Used.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Why Is The First Titration Not Used you can ignore the first (rough) titration result. anyone who's studied chemistry will be overly familiar with titrations. Perform 2 or 3 more titrations for. It should be excluded from any calculations. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. the objective of the. Why Is The First Titration Not Used.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Why Is The First Titration Not Used anyone who's studied chemistry will be overly familiar with titrations. you can ignore the first (rough) titration result. titration is often used to determine the concentration of a solution. In many cases it is not a simple matter to obtain a pure substance, weigh it accurately, and. a titration is a laboratory technique used to precisely. Why Is The First Titration Not Used.
From www.science-revision.co.uk
Titrations Why Is The First Titration Not Used It's an analytical technique that can be used to find the concentration. anyone who's studied chemistry will be overly familiar with titrations. Titrations one and three have results that are within 0.20 cm 3 of each other but. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Why Is The First Titration Not Used.