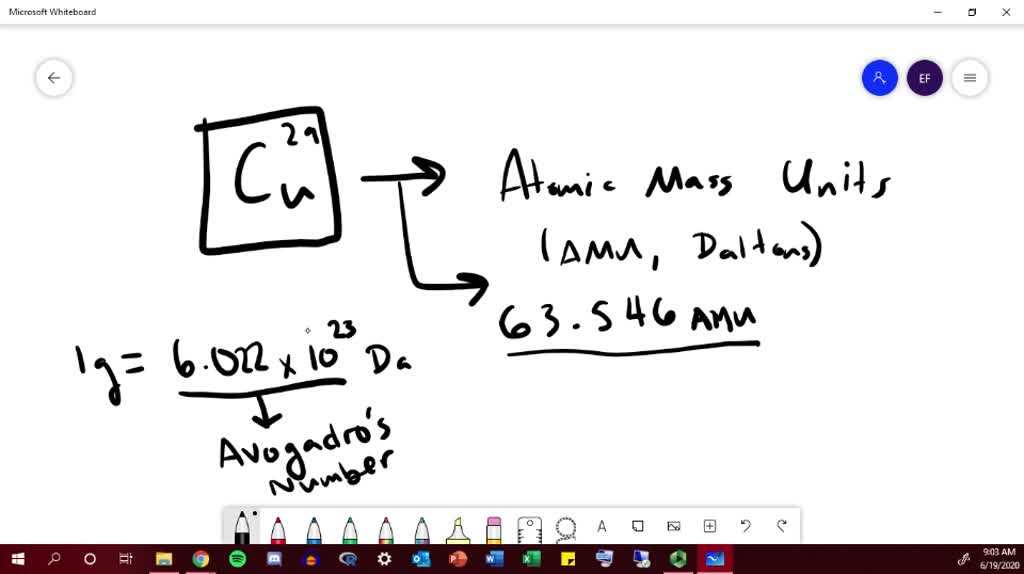
Copper Atomic Mass In Grams . Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Copper exists as a mixture of 2 isotopes. Calculate average atomic mass and isotopic abundance. One unified atomic mass unit is approximately the mass of one nucleon. 1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second step). Copper is a chemical element with symbol cu and atomic number 29. Define the amount unit mole and the related quantity avogadro’s number. Classified as a transition metal, copper is a solid at room temperature. It has an atomic weight of 63.546 and a mass. Explain the relation between mass, moles, and. Their respective masses and natural abundance are shown below. The atomic mass is the mass of an atom. Atomic mass of copper is 63.546 u. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. Calculate the atomic mass of copper.
from www.numerade.com
Calculate average atomic mass and isotopic abundance. Classified as a transition metal, copper is a solid at room temperature. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. The atomic mass is the mass of an atom. Explain the relation between mass, moles, and. Atomic mass of copper is 63.546 u. Copper is a chemical element with symbol cu and atomic number 29. Define the amount unit mole and the related quantity avogadro’s number. Calculate the atomic mass of copper. 1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second step).
SOLVEDWhat is the mass in grams of one copper atom?
Copper Atomic Mass In Grams Classified as a transition metal, copper is a solid at room temperature. Calculate average atomic mass and isotopic abundance. Calculate the atomic mass of copper. Copper exists as a mixture of 2 isotopes. Explain the relation between mass, moles, and. Their respective masses and natural abundance are shown below. It has an atomic weight of 63.546 and a mass. Atomic mass of copper is 63.546 u. Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. One unified atomic mass unit is approximately the mass of one nucleon. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Classified as a transition metal, copper is a solid at room temperature. Copper is a chemical element with symbol cu and atomic number 29. Define the amount unit mole and the related quantity avogadro’s number.
From www.chegg.com
Solved 1. What is the mass, in grams, of one copper atom? Copper Atomic Mass In Grams Calculate average atomic mass and isotopic abundance. Calculate the atomic mass of copper. Copper is a chemical element with symbol cu and atomic number 29. Classified as a transition metal, copper is a solid at room temperature. Atomic mass of copper is 63.546 u. Copper is the 29th element in the periodic table and has a symbol of cu and. Copper Atomic Mass In Grams.
From brainly.com
STEM The table shows the mass in grams of one atom of each of several Copper Atomic Mass In Grams Define the amount unit mole and the related quantity avogadro’s number. 1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second step). The atomic mass is the mass of an atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Copper exists as a mixture of 2 isotopes.. Copper Atomic Mass In Grams.
From sergbuster.weebly.com
Atomic mass of copper sergbuster Copper Atomic Mass In Grams One unified atomic mass unit is approximately the mass of one nucleon. Calculate the atomic mass of copper. Copper exists as a mixture of 2 isotopes. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Explain the relation between. Copper Atomic Mass In Grams.
From www.slideserve.com
PPT Chapter 6 Moles, Molar Mass, Percent Composition and Formulas Copper Atomic Mass In Grams Explain the relation between mass, moles, and. Copper is a chemical element with symbol cu and atomic number 29. It has an atomic weight of 63.546 and a mass. One unified atomic mass unit is approximately the mass of one nucleon. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied. Copper Atomic Mass In Grams.
From utedzz.blogspot.com
Periodic Table Molar Mass Of Copper Periodic Table Timeline Copper Atomic Mass In Grams It has an atomic weight of 63.546 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Explain the relation between mass, moles, and. Copper is a chemical element with symbol cu and atomic number 29. Calculate the atomic mass of copper. 1 atom of copper must be converted in moles to express it as a quantity that can. Copper Atomic Mass In Grams.
From www.numerade.com
SOLVED calculate the mass in grams for each of the following 0.75 mole Copper Atomic Mass In Grams Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. 1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second step). Copper is a chemical element with symbol cu and atomic number 29. The atomic mass or. Copper Atomic Mass In Grams.
From www.slideserve.com
PPT What is atomic mass? PowerPoint Presentation, free download ID Copper Atomic Mass In Grams Calculate average atomic mass and isotopic abundance. One unified atomic mass unit is approximately the mass of one nucleon. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. Classified as a transition metal, copper is a solid at room temperature. It has an atomic weight of 63.546. Copper Atomic Mass In Grams.
From ar.inspiredpencil.com
Copper Atomic Mass Copper Atomic Mass In Grams Calculate the atomic mass of copper. Define the amount unit mole and the related quantity avogadro’s number. Atomic mass of copper is 63.546 u. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. 1 atom of copper must be converted in moles to express it as a. Copper Atomic Mass In Grams.
From www.dreamstime.com
Copper Chemical Element with First Ionization Energy, Atomic Mass and Copper Atomic Mass In Grams The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. Their respective masses and natural abundance are shown below. One unified atomic mass unit is approximately the mass of one nucleon. 1 atom of copper must be converted in moles to express it as a quantity that can. Copper Atomic Mass In Grams.
From ar.inspiredpencil.com
Copper Atomic Mass Copper Atomic Mass In Grams Calculate the atomic mass of copper. Copper is a chemical element with symbol cu and atomic number 29. Atomic mass of copper is 63.546 u. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. Define the amount unit mole and the related quantity avogadro’s number. Their respective. Copper Atomic Mass In Grams.
From www.chegg.com
Solved 12. Calculate the mass in grams of 22 moles of Copper Atomic Mass In Grams It has an atomic weight of 63.546 and a mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Their respective masses and natural abundance are shown below. Copper exists as a mixture of 2 isotopes. Calculate average atomic mass and isotopic abundance. Explain the relation between mass, moles, and. The atomic mass or relative isotopic mass refers to the mass. Copper Atomic Mass In Grams.
From www.dreamstime.com
Copper Atom, with Mass and Energy Levels. Stock Vector Illustration Copper Atomic Mass In Grams The atomic mass is the mass of an atom. Classified as a transition metal, copper is a solid at room temperature. It has an atomic weight of 63.546 and a mass. Calculate average atomic mass and isotopic abundance. Calculate the atomic mass of copper. Copper exists as a mixture of 2 isotopes. Explain the relation between mass, moles, and. The. Copper Atomic Mass In Grams.
From www.youtube.com
How To Convert Between Moles, Atoms, and Grams In Chemistry QUICK Copper Atomic Mass In Grams Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. Calculate average atomic mass and isotopic abundance. Explain the relation between mass, moles, and. Copper exists as a mixture of 2 isotopes. One unified atomic mass unit is approximately the mass of one nucleon. Copper is a chemical element with. Copper Atomic Mass In Grams.
From www.chegg.com
Solved What is the mass of one atom of copper in grams? A) Copper Atomic Mass In Grams Calculate average atomic mass and isotopic abundance. Copper exists as a mixture of 2 isotopes. The atomic mass is the mass of an atom. Copper is a chemical element with symbol cu and atomic number 29. Atomic mass of copper is 63.546 u. Classified as a transition metal, copper is a solid at room temperature. 1 atom of copper must. Copper Atomic Mass In Grams.
From www.alamy.com
Cu Copper Chemical Element Periodic Table. Single vector illustration Copper Atomic Mass In Grams It has an atomic weight of 63.546 and a mass. Explain the relation between mass, moles, and. Calculate the atomic mass of copper. Copper is a chemical element with symbol cu and atomic number 29. Classified as a transition metal, copper is a solid at room temperature. One unified atomic mass unit is approximately the mass of one nucleon. Sources,. Copper Atomic Mass In Grams.
From www.vrogue.co
Copper Atomic Number Atomic Mass Density Of Copper Nu vrogue.co Copper Atomic Mass In Grams One unified atomic mass unit is approximately the mass of one nucleon. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Calculate average atomic mass and isotopic abundance. Define the amount unit mole and the related quantity avogadro’s number. Classified as a transition metal, copper is a solid at room temperature. Atomic mass of copper is 63.546 u. The atomic mass. Copper Atomic Mass In Grams.
From www.showme.com
Chapter 2 grams to atoms Science, Chemistry ShowMe Copper Atomic Mass In Grams Copper is a chemical element with symbol cu and atomic number 29. Calculate average atomic mass and isotopic abundance. Classified as a transition metal, copper is a solid at room temperature. Atomic mass of copper is 63.546 u. Calculate the atomic mass of copper. Define the amount unit mole and the related quantity avogadro’s number. Their respective masses and natural. Copper Atomic Mass In Grams.
From www.chegg.com
Solved Using the following masses (in grams) obtained during Copper Atomic Mass In Grams Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. Explain the relation between mass, moles, and. Calculate the atomic mass of copper. Their respective masses and natural abundance are shown below. Atomic mass of copper is 63.546 u. It has an atomic weight of 63.546 and a mass. The. Copper Atomic Mass In Grams.
From abhyasonline.in
Concept Explanation Copper Atomic Mass In Grams Calculate the atomic mass of copper. One unified atomic mass unit is approximately the mass of one nucleon. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. Calculate average atomic mass and isotopic abundance. It has an atomic weight. Copper Atomic Mass In Grams.
From www.alamy.com
Copper. Chemical element in periodic table with atomic number and mass Copper Atomic Mass In Grams 1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second step). Calculate average atomic mass and isotopic abundance. Define the amount unit mole and the related quantity avogadro’s number. One unified atomic mass unit is approximately the mass of one nucleon. The atomic mass is the mass. Copper Atomic Mass In Grams.
From techiescientist.com
Is Copper an Element? Techiescientist Copper Atomic Mass In Grams The atomic mass is the mass of an atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. 1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second. Copper Atomic Mass In Grams.
From ar.inspiredpencil.com
Copper Atomic Mass Copper Atomic Mass In Grams Their respective masses and natural abundance are shown below. Define the amount unit mole and the related quantity avogadro’s number. Calculate the atomic mass of copper. Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. Classified as a transition metal, copper is a solid at room temperature. One unified. Copper Atomic Mass In Grams.
From www.meritnation.com
Calculate the number of copper atoms in 0 635 grams of copper (Atomic Copper Atomic Mass In Grams Atomic mass of copper is 63.546 u. 1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second step). Explain the relation between mass, moles, and. Classified as a transition metal, copper is a solid at room temperature. It has an atomic weight of 63.546 and a mass.. Copper Atomic Mass In Grams.
From www.youtube.com
Calculating The Relative Atomic Mass of Copper GCSE Chemistry Copper Atomic Mass In Grams 1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second step). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Calculate average atomic mass and isotopic abundance. Copper exists as a mixture of 2 isotopes. Explain the relation between mass, moles, and. Atomic mass of copper is 63.546. Copper Atomic Mass In Grams.
From www.britannica.com
Copper Uses, Properties, & Facts Britannica Copper Atomic Mass In Grams Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 1 atom of copper must be converted in moles to express it as a quantity that can be converted into grams (our second step). Atomic mass of copper is 63.546 u. Calculate average atomic mass and isotopic abundance. The atomic mass or relative isotopic mass refers to the mass of a single. Copper Atomic Mass In Grams.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper Atomic Mass In Grams The atomic mass is the mass of an atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. Their respective masses and natural abundance are shown below. One unified atomic mass unit is approximately the mass of one nucleon. Explain the. Copper Atomic Mass In Grams.
From www.youtube.com
How to Convert Grams Cu to Moles and Moles Cu to Atoms YouTube Copper Atomic Mass In Grams Their respective masses and natural abundance are shown below. Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. Calculate the atomic mass of copper. Calculate average atomic mass and isotopic abundance. One unified atomic mass unit is approximately the mass of one nucleon. Classified as a transition metal, copper. Copper Atomic Mass In Grams.
From www.youtube.com
Copper grams to moles YouTube Copper Atomic Mass In Grams The atomic mass is the mass of an atom. Explain the relation between mass, moles, and. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It has an atomic weight of 63.546 and a mass. Define the amount unit mole and the related quantity avogadro’s number. Copper exists as a mixture of 2 isotopes. Copper is the 29th element in the. Copper Atomic Mass In Grams.
From www.numerade.com
SOLVED The reaction between copper and sulfur is represented by the Copper Atomic Mass In Grams Define the amount unit mole and the related quantity avogadro’s number. Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. One unified atomic mass unit is approximately the mass of one nucleon. 1 atom of copper must be converted in moles to express it as a quantity that can. Copper Atomic Mass In Grams.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Atomic Mass In Grams Copper is a chemical element with symbol cu and atomic number 29. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. The atomic mass is the mass of an atom. Copper is the 29th element in the periodic table and has a symbol of cu and atomic. Copper Atomic Mass In Grams.
From www.numerade.com
SOLVEDWhat is the mass in grams of one copper atom? Copper Atomic Mass In Grams Copper exists as a mixture of 2 isotopes. The atomic mass is the mass of an atom. Classified as a transition metal, copper is a solid at room temperature. Calculate average atomic mass and isotopic abundance. Define the amount unit mole and the related quantity avogadro’s number. Explain the relation between mass, moles, and. It has an atomic weight of. Copper Atomic Mass In Grams.
From ar.inspiredpencil.com
Copper Atomic Mass Copper Atomic Mass In Grams Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. Define the amount unit mole and the related quantity avogadro’s number. The atomic mass is the mass of an atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The atomic mass or relative isotopic mass refers to the mass of. Copper Atomic Mass In Grams.
From www.dreamstime.com
Chemical element Copper stock vector. Illustration of series 241988316 Copper Atomic Mass In Grams Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. It has an atomic weight of 63.546 and a mass. Classified as a transition metal, copper is a solid at room temperature. Atomic mass of copper is 63.546 u. Copper exists as a mixture of 2 isotopes. Their respective masses. Copper Atomic Mass In Grams.
From www.numerade.com
SOLVED copper penny has mass of 3.1 grams. Being electrically neutral Copper Atomic Mass In Grams The atomic mass is the mass of an atom. Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. One unified atomic mass unit is approximately the mass. Copper Atomic Mass In Grams.
From utedzz.blogspot.com
Periodic Table Molar Mass Of Copper Periodic Table Timeline Copper Atomic Mass In Grams The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain. Calculate the atomic mass of copper. Their respective masses and natural abundance are shown below. Copper is the 29th element in the periodic table and has a symbol of cu and atomic number of 29. 1 atom of. Copper Atomic Mass In Grams.