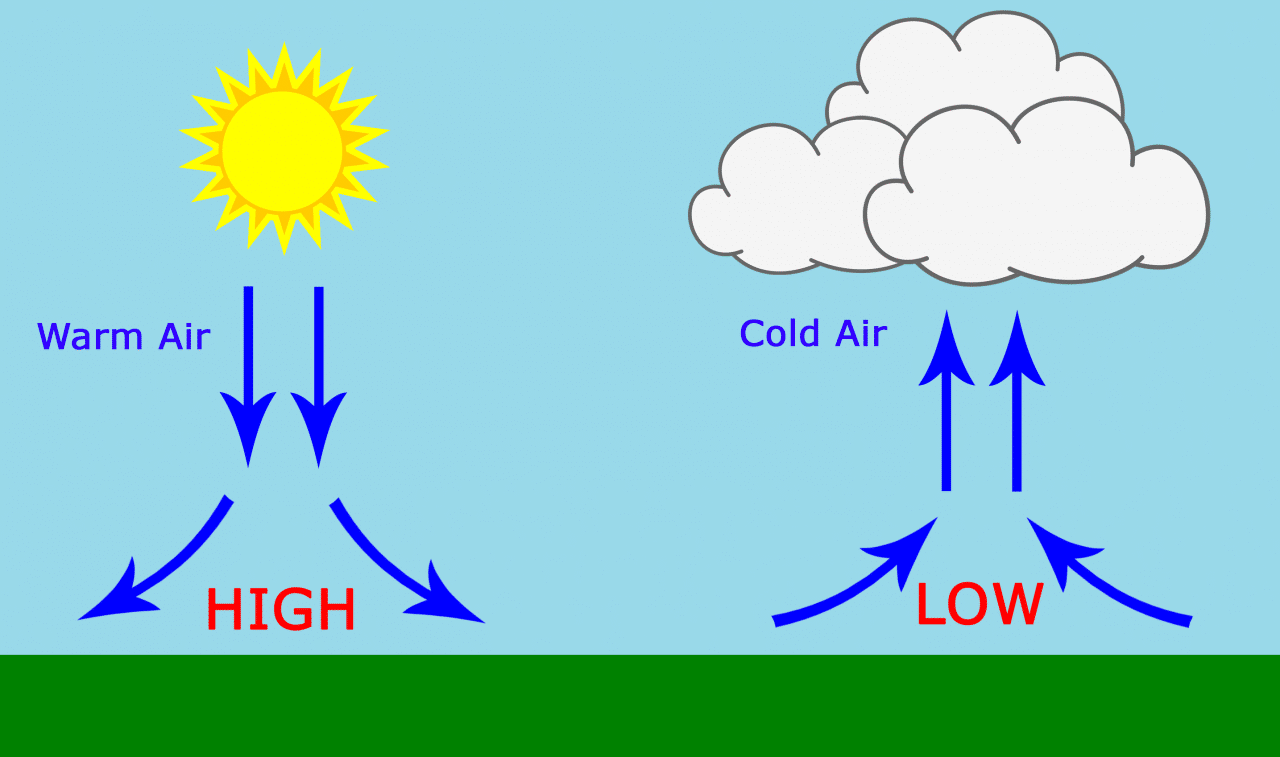
Do Gases Move From High Pressure To Low Pressure . They can move from a low pressure region to high. particle do not always move from high pressure to low pressure. actually it's very rare that air moves straightly from high to low pressure: More frequently it goes on spiraling around high or low pressure. places where the air pressure is high, are called high pressure systems. A low pressure system has lower pressure at its center than the areas around it. air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. why do real gases behave so differently from ideal gases at high pressures and low temperatures? when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. Under these conditions, the two basic.
from m7et.com
when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. A low pressure system has lower pressure at its center than the areas around it. Under these conditions, the two basic. why do real gases behave so differently from ideal gases at high pressures and low temperatures? More frequently it goes on spiraling around high or low pressure. They can move from a low pressure region to high. places where the air pressure is high, are called high pressure systems. actually it's very rare that air moves straightly from high to low pressure: particle do not always move from high pressure to low pressure. air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy.
بحث كامل عن الضغط الجوي واشهر 5 واحدت لقياسه
Do Gases Move From High Pressure To Low Pressure when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. actually it's very rare that air moves straightly from high to low pressure: air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. More frequently it goes on spiraling around high or low pressure. A low pressure system has lower pressure at its center than the areas around it. places where the air pressure is high, are called high pressure systems. why do real gases behave so differently from ideal gases at high pressures and low temperatures? They can move from a low pressure region to high. when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. Under these conditions, the two basic. particle do not always move from high pressure to low pressure.
From www.slideserve.com
PPT High and Low Pressure Systems Weather Systems Unit PowerPoint Do Gases Move From High Pressure To Low Pressure when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. Under these conditions, the two basic. They can move from a low pressure region to high. actually it's very rare that air moves straightly from high to low pressure: particle do not always. Do Gases Move From High Pressure To Low Pressure.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Do Gases Move From High Pressure To Low Pressure actually it's very rare that air moves straightly from high to low pressure: A low pressure system has lower pressure at its center than the areas around it. Under these conditions, the two basic. air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. They can move. Do Gases Move From High Pressure To Low Pressure.
From sciencenotes.org
Real Gas vs Ideal Gas Do Gases Move From High Pressure To Low Pressure why do real gases behave so differently from ideal gases at high pressures and low temperatures? air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. A low pressure system has lower pressure at its center than the areas around it. places where the air pressure. Do Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT Weather Vocabulary Terms PowerPoint Presentation, free download Do Gases Move From High Pressure To Low Pressure They can move from a low pressure region to high. More frequently it goes on spiraling around high or low pressure. air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. why do real gases behave so differently from ideal gases at high pressures and low temperatures?. Do Gases Move From High Pressure To Low Pressure.
From slideplayer.com
High and Low Pressure Systems Weather Systems Unit ppt download Do Gases Move From High Pressure To Low Pressure air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. why do real gases behave so differently from ideal gases at high pressures and low temperatures? They can move from a low pressure region to high. More frequently it goes on spiraling around high or low pressure.. Do Gases Move From High Pressure To Low Pressure.
From www.logiota.com
Deviation from Ideal Gas Behavior States of Matter Physical Do Gases Move From High Pressure To Low Pressure More frequently it goes on spiraling around high or low pressure. Under these conditions, the two basic. places where the air pressure is high, are called high pressure systems. They can move from a low pressure region to high. actually it's very rare that air moves straightly from high to low pressure: air is a fluid (even. Do Gases Move From High Pressure To Low Pressure.
From exokrimdo.blob.core.windows.net
Why Do Gases Move From High Pressure To Low Pressure at Mark Jones blog Do Gases Move From High Pressure To Low Pressure Under these conditions, the two basic. actually it's very rare that air moves straightly from high to low pressure: More frequently it goes on spiraling around high or low pressure. places where the air pressure is high, are called high pressure systems. air is a fluid (even though it isn't a liquid) and so will flow into. Do Gases Move From High Pressure To Low Pressure.
From exokrimdo.blob.core.windows.net
Why Do Gases Move From High Pressure To Low Pressure at Mark Jones blog Do Gases Move From High Pressure To Low Pressure places where the air pressure is high, are called high pressure systems. when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. actually it's very rare that air moves straightly from high to low pressure: air is a fluid (even though it. Do Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT The Restless Atmosphere PowerPoint Presentation, free download Do Gases Move From High Pressure To Low Pressure They can move from a low pressure region to high. places where the air pressure is high, are called high pressure systems. when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. A low pressure system has lower pressure at its center than the. Do Gases Move From High Pressure To Low Pressure.
From www.thegeographeronline.net
Weather and Climate THE GEOGRAPHER ONLINE Do Gases Move From High Pressure To Low Pressure why do real gases behave so differently from ideal gases at high pressures and low temperatures? Under these conditions, the two basic. actually it's very rare that air moves straightly from high to low pressure: places where the air pressure is high, are called high pressure systems. A low pressure system has lower pressure at its center. Do Gases Move From High Pressure To Low Pressure.
From ar.inspiredpencil.com
High And Low Pressure Systems Do Gases Move From High Pressure To Low Pressure A low pressure system has lower pressure at its center than the areas around it. air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. places where the air pressure is high, are called high pressure systems. when the two containers are put side to side. Do Gases Move From High Pressure To Low Pressure.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Do Gases Move From High Pressure To Low Pressure places where the air pressure is high, are called high pressure systems. They can move from a low pressure region to high. air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. particle do not always move from high pressure to low pressure. actually it's. Do Gases Move From High Pressure To Low Pressure.
From learnbps.bismarckschools.org
States of Matter (Book) Behavior of Gases Learnbps Do Gases Move From High Pressure To Low Pressure particle do not always move from high pressure to low pressure. places where the air pressure is high, are called high pressure systems. when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. A low pressure system has lower pressure at its center. Do Gases Move From High Pressure To Low Pressure.
From fyospmjln.blob.core.windows.net
High And Low Air Pressure Symbols at Brian Chamberlin blog Do Gases Move From High Pressure To Low Pressure actually it's very rare that air moves straightly from high to low pressure: More frequently it goes on spiraling around high or low pressure. Under these conditions, the two basic. when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. A low pressure system. Do Gases Move From High Pressure To Low Pressure.
From pilotinstitute.com
High vs. LowPressure Systems Explained Pilot Institute Do Gases Move From High Pressure To Low Pressure A low pressure system has lower pressure at its center than the areas around it. More frequently it goes on spiraling around high or low pressure. They can move from a low pressure region to high. when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles. Do Gases Move From High Pressure To Low Pressure.
From www.britannica.com
perfect gas law chemistry and physics Britannica Do Gases Move From High Pressure To Low Pressure particle do not always move from high pressure to low pressure. More frequently it goes on spiraling around high or low pressure. Under these conditions, the two basic. why do real gases behave so differently from ideal gases at high pressures and low temperatures? when the two containers are put side to side and gas is allowed. Do Gases Move From High Pressure To Low Pressure.
From www.edplace.com
Understand Gas Pressure Worksheet EdPlace Do Gases Move From High Pressure To Low Pressure when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. particle do not always move from high pressure to low pressure. Under these conditions, the two basic. places where the air pressure is high, are called high pressure systems. air is a. Do Gases Move From High Pressure To Low Pressure.
From owlcation.com
The Theories and Behavior of Gas Owlcation Do Gases Move From High Pressure To Low Pressure actually it's very rare that air moves straightly from high to low pressure: when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. Under these conditions, the two basic. They can move from a low pressure region to high. More frequently it goes on. Do Gases Move From High Pressure To Low Pressure.
From www.expii.com
Real vs. Ideal Gases — Comparison & Importance Expii Do Gases Move From High Pressure To Low Pressure Under these conditions, the two basic. A low pressure system has lower pressure at its center than the areas around it. particle do not always move from high pressure to low pressure. places where the air pressure is high, are called high pressure systems. More frequently it goes on spiraling around high or low pressure. why do. Do Gases Move From High Pressure To Low Pressure.
From saylordotorg.github.io
Gases Do Gases Move From High Pressure To Low Pressure More frequently it goes on spiraling around high or low pressure. why do real gases behave so differently from ideal gases at high pressures and low temperatures? particle do not always move from high pressure to low pressure. A low pressure system has lower pressure at its center than the areas around it. when the two containers. Do Gases Move From High Pressure To Low Pressure.
From www.istockphoto.com
High Pressure And Low Pressure Illustration Stock Illustration Do Gases Move From High Pressure To Low Pressure places where the air pressure is high, are called high pressure systems. actually it's very rare that air moves straightly from high to low pressure: They can move from a low pressure region to high. why do real gases behave so differently from ideal gases at high pressures and low temperatures? when the two containers are. Do Gases Move From High Pressure To Low Pressure.
From exokrimdo.blob.core.windows.net
Why Do Gases Move From High Pressure To Low Pressure at Mark Jones blog Do Gases Move From High Pressure To Low Pressure More frequently it goes on spiraling around high or low pressure. Under these conditions, the two basic. air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. A low pressure system has lower pressure at its center than the areas around it. actually it's very rare that. Do Gases Move From High Pressure To Low Pressure.
From www.youtube.com
Does fluid always flow from high pressure to low pressure? Quick Do Gases Move From High Pressure To Low Pressure why do real gases behave so differently from ideal gases at high pressures and low temperatures? when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. Under these conditions, the two basic. More frequently it goes on spiraling around high or low pressure. . Do Gases Move From High Pressure To Low Pressure.
From www.ixl.com
IXL How does particle motion affect gas pressure? 8th grade science Do Gases Move From High Pressure To Low Pressure More frequently it goes on spiraling around high or low pressure. places where the air pressure is high, are called high pressure systems. actually it's very rare that air moves straightly from high to low pressure: air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy.. Do Gases Move From High Pressure To Low Pressure.
From pilotinstitute.com
High vs. LowPressure Systems Explained Pilot Institute Do Gases Move From High Pressure To Low Pressure A low pressure system has lower pressure at its center than the areas around it. places where the air pressure is high, are called high pressure systems. actually it's very rare that air moves straightly from high to low pressure: when the two containers are put side to side and gas is allowed to flow, the full,. Do Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT Atmospheric Motion PowerPoint Presentation, free download ID Do Gases Move From High Pressure To Low Pressure places where the air pressure is high, are called high pressure systems. when the two containers are put side to side and gas is allowed to flow, the full, high pressure container will lose particles to. actually it's very rare that air moves straightly from high to low pressure: They can move from a low pressure region. Do Gases Move From High Pressure To Low Pressure.
From saylordotorg.github.io
Gases and Pressure Do Gases Move From High Pressure To Low Pressure actually it's very rare that air moves straightly from high to low pressure: Under these conditions, the two basic. why do real gases behave so differently from ideal gases at high pressures and low temperatures? More frequently it goes on spiraling around high or low pressure. particle do not always move from high pressure to low pressure.. Do Gases Move From High Pressure To Low Pressure.
From www.visionlearning.com
Properties of Gases Chemistry Visionlearning Do Gases Move From High Pressure To Low Pressure More frequently it goes on spiraling around high or low pressure. They can move from a low pressure region to high. why do real gases behave so differently from ideal gases at high pressures and low temperatures? Under these conditions, the two basic. places where the air pressure is high, are called high pressure systems. particle do. Do Gases Move From High Pressure To Low Pressure.
From www.slideserve.com
PPT Chapter 14 The Behavior of Gases PowerPoint Presentation, free Do Gases Move From High Pressure To Low Pressure Under these conditions, the two basic. More frequently it goes on spiraling around high or low pressure. particle do not always move from high pressure to low pressure. A low pressure system has lower pressure at its center than the areas around it. air is a fluid (even though it isn't a liquid) and so will flow into. Do Gases Move From High Pressure To Low Pressure.
From exokrimdo.blob.core.windows.net
Why Do Gases Move From High Pressure To Low Pressure at Mark Jones blog Do Gases Move From High Pressure To Low Pressure air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. why do real gases behave so differently from ideal gases at high pressures and low temperatures? More frequently it goes on spiraling around high or low pressure. when the two containers are put side to side. Do Gases Move From High Pressure To Low Pressure.
From giokwafes.blob.core.windows.net
How Does Pressure Move From High To Low at Larry Brown blog Do Gases Move From High Pressure To Low Pressure actually it's very rare that air moves straightly from high to low pressure: particle do not always move from high pressure to low pressure. A low pressure system has lower pressure at its center than the areas around it. More frequently it goes on spiraling around high or low pressure. why do real gases behave so differently. Do Gases Move From High Pressure To Low Pressure.
From m7et.com
بحث كامل عن الضغط الجوي واشهر 5 واحدت لقياسه Do Gases Move From High Pressure To Low Pressure places where the air pressure is high, are called high pressure systems. They can move from a low pressure region to high. why do real gases behave so differently from ideal gases at high pressures and low temperatures? More frequently it goes on spiraling around high or low pressure. air is a fluid (even though it isn't. Do Gases Move From High Pressure To Low Pressure.
From www.askiitians.com
Gas Laws And Properties Of Gases Study Material for IIT JEE askIITians Do Gases Move From High Pressure To Low Pressure air is a fluid (even though it isn't a liquid) and so will flow into areas where it can expend energy. why do real gases behave so differently from ideal gases at high pressures and low temperatures? A low pressure system has lower pressure at its center than the areas around it. Under these conditions, the two basic.. Do Gases Move From High Pressure To Low Pressure.
From www.myarklamiss.com
HIGH vs. LOW What are the impacts of weather pressures? KTVE Do Gases Move From High Pressure To Low Pressure actually it's very rare that air moves straightly from high to low pressure: A low pressure system has lower pressure at its center than the areas around it. Under these conditions, the two basic. More frequently it goes on spiraling around high or low pressure. why do real gases behave so differently from ideal gases at high pressures. Do Gases Move From High Pressure To Low Pressure.
From www.fox6now.com
The physics behind high and low pressure FOX6 Milwaukee Do Gases Move From High Pressure To Low Pressure They can move from a low pressure region to high. Under these conditions, the two basic. particle do not always move from high pressure to low pressure. actually it's very rare that air moves straightly from high to low pressure: places where the air pressure is high, are called high pressure systems. More frequently it goes on. Do Gases Move From High Pressure To Low Pressure.