Lead And Ammonium Hydroxide Reaction . compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. Recognize and identify examples of precipitation reactions. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Apply the solubility rules of common. use the calculator below to balance chemical equations and determine the type of reaction (instructions).
from www.slideshare.net
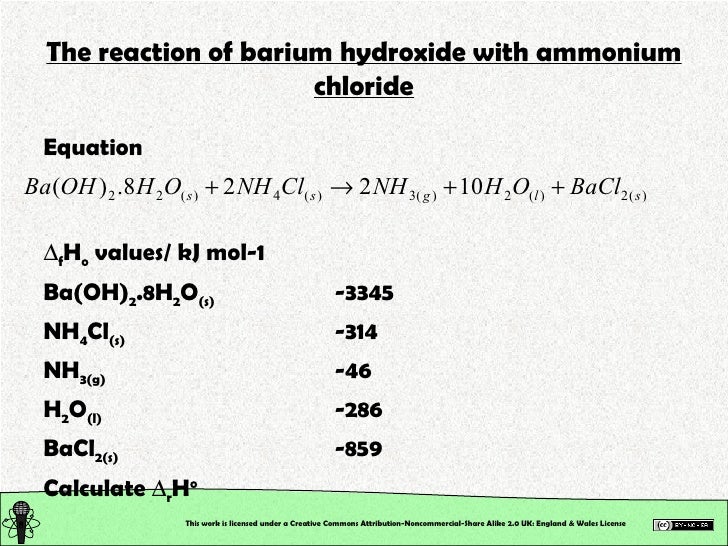
enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Apply the solubility rules of common. Recognize and identify examples of precipitation reactions. use the calculator below to balance chemical equations and determine the type of reaction (instructions). ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions:
Chemical Reactions Thermochemistry
Lead And Ammonium Hydroxide Reaction ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. use the calculator below to balance chemical equations and determine the type of reaction (instructions). Recognize and identify examples of precipitation reactions. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Apply the solubility rules of common. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations.
From www.toppr.com
(51 Write the balanced chemical equations Lead sulphate + ammonium Lead And Ammonium Hydroxide Reaction in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. Apply the solubility rules of common. use the calculator below to balance chemical equations. Lead And Ammonium Hydroxide Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for NH3 + H2SO4 = (NH4)2SO4 YouTube Lead And Ammonium Hydroxide Reaction use the calculator below to balance chemical equations and determine the type of reaction (instructions). compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Recognize and identify examples of. Lead And Ammonium Hydroxide Reaction.
From www.youtube.com
Reaction of Copper Sulfate with Ammonium Hydroxide / Ammonia CuSO4 Lead And Ammonium Hydroxide Reaction ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Recognize and identify examples of precipitation reactions. compare the colours of lead compounds formed by precipitation reactions to. Lead And Ammonium Hydroxide Reaction.
From mariabsmitho.blob.core.windows.net
Lead Hydroxide Reaction Equation at mariabsmitho blog Lead And Ammonium Hydroxide Reaction ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. Apply the solubility rules of common. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: enter an ionic equation (solubility states are optional). Lead And Ammonium Hydroxide Reaction.
From www.researchgate.net
What kind of reaction mechanism we expect for ammonium hydroxide with Lead And Ammonium Hydroxide Reaction in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: use the calculator below to balance chemical equations and determine the type of reaction (instructions). ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which. Lead And Ammonium Hydroxide Reaction.
From www.alamy.com
Labelled diagram to show the diffusion of ammonia and hydrogen Stock Lead And Ammonium Hydroxide Reaction in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2,. Lead And Ammonium Hydroxide Reaction.
From www.nagwa.com
Question Video Recalling the Correct Product Formed from the Reaction Lead And Ammonium Hydroxide Reaction compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. Recognize and identify examples of precipitation reactions. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Apply the solubility rules of common. ammonium hydroxide (. Lead And Ammonium Hydroxide Reaction.
From www.alamy.com
Ammonium hydroxide, ammonia solution, NH4OH molecule. Structural Lead And Ammonium Hydroxide Reaction in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. Apply the solubility rules of common. enter an ionic equation (solubility states are optional) to calculate. Lead And Ammonium Hydroxide Reaction.
From www.chegg.com
Solved 3. What are the total ionic equation and the net Lead And Ammonium Hydroxide Reaction in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. use the calculator below to balance chemical equations and determine the type of reaction. Lead And Ammonium Hydroxide Reaction.
From hubpages.com
Complete Chemistry of Hydrolysis & Hydration. HubPages Lead And Ammonium Hydroxide Reaction Apply the solubility rules of common. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. use the calculator below to balance chemical equations and determine the type of reaction. Lead And Ammonium Hydroxide Reaction.
From www.numerade.com
SOLVED The net ionic equation for the reaction of aqueous sodium Lead And Ammonium Hydroxide Reaction Apply the solubility rules of common. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: ammonium hydroxide ( nh 4 oh) reacts with lead nitrate. Lead And Ammonium Hydroxide Reaction.
From www.icseboards.com
Notes For ICSE Class 10 Chemistry Analytical Chemistry ICSE Board Lead And Ammonium Hydroxide Reaction use the calculator below to balance chemical equations and determine the type of reaction (instructions). compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. ammonium hydroxide ( nh. Lead And Ammonium Hydroxide Reaction.
From ar.inspiredpencil.com
Ammonium Hydroxide Structure Lead And Ammonium Hydroxide Reaction compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. use the calculator below to balance chemical equations and determine the type of reaction (instructions). enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. in the sections that. Lead And Ammonium Hydroxide Reaction.
From www.slideshare.net
Chemical Reactions Thermochemistry Lead And Ammonium Hydroxide Reaction ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. Recognize and identify examples of precipitation reactions. Apply the solubility rules of common. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. . Lead And Ammonium Hydroxide Reaction.
From fyojqwyda.blob.core.windows.net
Lead Hydroxide Reaction at Jonathan Ertl blog Lead And Ammonium Hydroxide Reaction in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Recognize and identify examples of precipitation reactions. Apply the solubility rules of common. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. use. Lead And Ammonium Hydroxide Reaction.
From ar.inspiredpencil.com
Lead Nitrate Solution Lead And Ammonium Hydroxide Reaction Apply the solubility rules of common. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Recognize and identify examples of precipitation reactions. compare the colours of lead compounds formed by. Lead And Ammonium Hydroxide Reaction.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记6.3.3 Reactions of Ions in Aqueous Lead And Ammonium Hydroxide Reaction in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: use the calculator below to balance chemical equations and determine the type of reaction (instructions). Recognize and identify examples of precipitation reactions. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white. Lead And Ammonium Hydroxide Reaction.
From fyojqwyda.blob.core.windows.net
Lead Hydroxide Reaction at Jonathan Ertl blog Lead And Ammonium Hydroxide Reaction Recognize and identify examples of precipitation reactions. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: use the calculator below to balance chemical equations and determine the type of reaction (instructions). ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white. Lead And Ammonium Hydroxide Reaction.
From www.wikihow.life
3 Ways to Study the Chemical Reactions of Ammonia wikiHow Life Lead And Ammonium Hydroxide Reaction Apply the solubility rules of common. use the calculator below to balance chemical equations and determine the type of reaction (instructions). compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. Recognize and identify examples of precipitation reactions. ammonium hydroxide ( nh 4 oh) reacts with. Lead And Ammonium Hydroxide Reaction.
From www.chemicals.co.uk
What is Ammonium Hydroxide? The Chemistry Blog Lead And Ammonium Hydroxide Reaction compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. Apply the solubility rules of common. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: enter an ionic equation (solubility states are optional) to calculate. Lead And Ammonium Hydroxide Reaction.
From www.numerade.com
Draw the structure of the quaternary ammonium hydroxide resulting from Lead And Ammonium Hydroxide Reaction compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. use the calculator below to balance chemical equations and determine the type of reaction (instructions). Apply the solubility rules of common. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic. Lead And Ammonium Hydroxide Reaction.
From www.researchgate.net
Schematic diagram showing how ammonium hydroxide catalyses the reaction Lead And Ammonium Hydroxide Reaction enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. Apply the solubility rules of common. use the calculator below to balance chemical equations and determine the type of reaction. Lead And Ammonium Hydroxide Reaction.
From www.youtube.com
Chemical Reaction of Ammonium Hydroxide and Silver Nitrate YouTube Lead And Ammonium Hydroxide Reaction enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. Apply the solubility rules of common. compare the colours of lead compounds formed by precipitation reactions to identify. Lead And Ammonium Hydroxide Reaction.
From www.numerade.com
SOLVED More Precise In Balancing Chemical Equations. Complete and Lead And Ammonium Hydroxide Reaction use the calculator below to balance chemical equations and determine the type of reaction (instructions). Recognize and identify examples of precipitation reactions. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this. Lead And Ammonium Hydroxide Reaction.
From chempedia.info
Water reaction with ammonia Big Chemical Encyclopedia Lead And Ammonium Hydroxide Reaction ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. Apply the solubility rules of common. use the calculator below to balance chemical equations and determine the type of reaction (instructions). in the sections that follow, we discuss three of the most important kinds. Lead And Ammonium Hydroxide Reaction.
From askfilo.com
3. Reaction of Ammonium Hydroxide with the Soluble Salt of Copper [Cu2+ i.. Lead And Ammonium Hydroxide Reaction compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions:. Lead And Ammonium Hydroxide Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for Pb(NO3)2 + (NH4)2CO3 = PbCO3 Lead And Ammonium Hydroxide Reaction Recognize and identify examples of precipitation reactions. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Apply the solubility rules of common. compare. Lead And Ammonium Hydroxide Reaction.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Lead And Ammonium Hydroxide Reaction use the calculator below to balance chemical equations and determine the type of reaction (instructions). Recognize and identify examples of precipitation reactions. Apply the solubility rules of common. enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce. Lead And Ammonium Hydroxide Reaction.
From courses.lumenlearning.com
Classifying Chemical Reactions Chemistry Lead And Ammonium Hydroxide Reaction Recognize and identify examples of precipitation reactions. Apply the solubility rules of common. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. use the calculator below to balance chemical equations. Lead And Ammonium Hydroxide Reaction.
From studylib.net
Analytical Chemistry (Use of NaOH and NH4OH) Lead And Ammonium Hydroxide Reaction Apply the solubility rules of common. compare the colours of lead compounds formed by precipitation reactions to identify which would make good pigments in this microscale class. use the calculator below to balance chemical equations and determine the type of reaction (instructions). ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white. Lead And Ammonium Hydroxide Reaction.
From www.researchgate.net
Ammonium hydroxide flocculation. Download Scientific Diagram Lead And Ammonium Hydroxide Reaction use the calculator below to balance chemical equations and determine the type of reaction (instructions). enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Recognize and identify examples of precipitation. Lead And Ammonium Hydroxide Reaction.
From www.slideserve.com
PPT Theoretical Yield Which Reactant is Limiting? PowerPoint Lead And Ammonium Hydroxide Reaction Recognize and identify examples of precipitation reactions. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: use the calculator below to balance chemical. Lead And Ammonium Hydroxide Reaction.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead(II) nitrate reacts Lead And Ammonium Hydroxide Reaction use the calculator below to balance chemical equations and determine the type of reaction (instructions). Apply the solubility rules of common. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. enter an ionic equation (solubility states are optional) to calculate its complete and. Lead And Ammonium Hydroxide Reaction.
From www.numerade.com
SOLVED Complete the following instructions for the problems below I Lead And Ammonium Hydroxide Reaction Recognize and identify examples of precipitation reactions. in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Apply the solubility rules of common. ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white precipitate of pb ( oh) 2, which is. compare. Lead And Ammonium Hydroxide Reaction.
From www.youtube.com
What happens when ammonium hydroxide reacts with sulphuric acid Lead And Ammonium Hydroxide Reaction in the sections that follow, we discuss three of the most important kinds of reactions that occur in aqueous solutions: Recognize and identify examples of precipitation reactions. use the calculator below to balance chemical equations and determine the type of reaction (instructions). ammonium hydroxide ( nh 4 oh) reacts with lead nitrate solution to produce a white. Lead And Ammonium Hydroxide Reaction.