How To Calculate The Concentration From A Titration . In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. the amount of added titrant is determined from its concentration and volume: let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. the process of calculating concentration from titration data is described and illustrated. titration is a method to determine the unknown concentration of a specific substance. to calculate concentration from titration, you can use the formula: if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. C1v1 = c2v2 where c1 is the concentration of the titrant (the.
from www.youtube.com
titration is a method to determine the unknown concentration of a specific substance. C1v1 = c2v2 where c1 is the concentration of the titrant (the. the amount of added titrant is determined from its concentration and volume: the process of calculating concentration from titration data is described and illustrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. to calculate concentration from titration, you can use the formula: if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh).
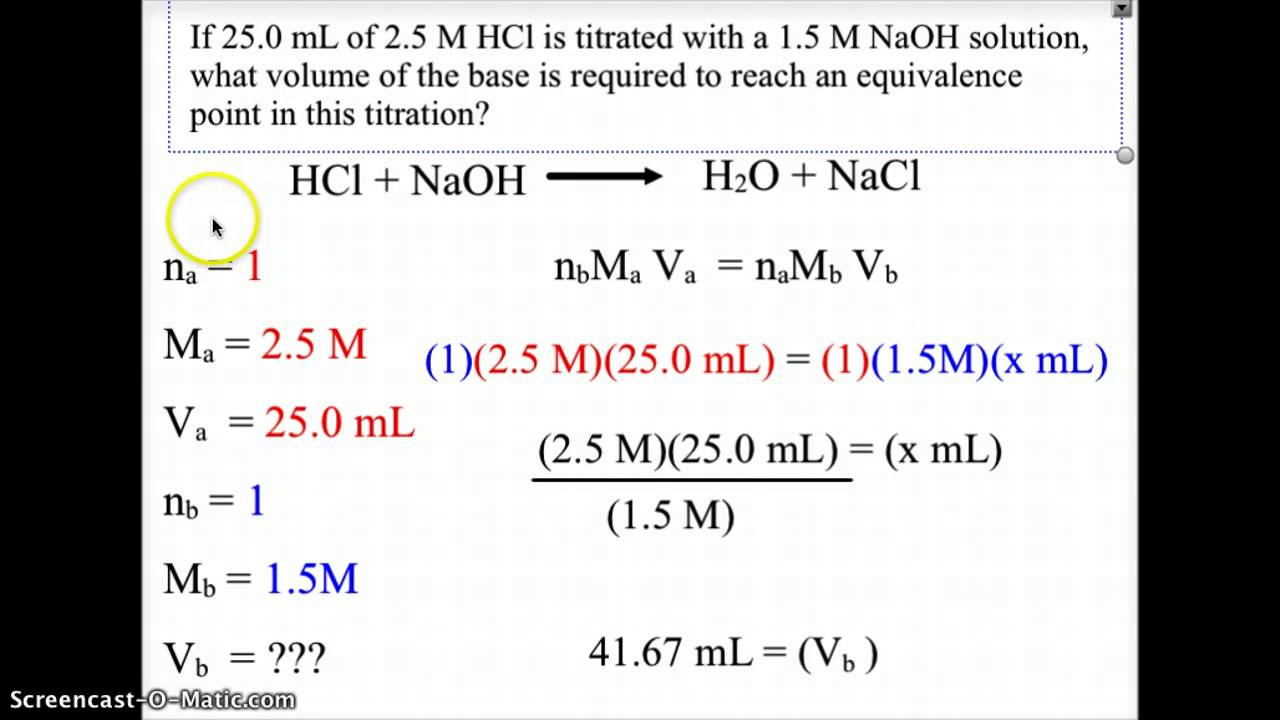
AcidBase Titration Equivalence Point YouTube
How To Calculate The Concentration From A Titration the process of calculating concentration from titration data is described and illustrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. the process of calculating concentration from titration data is described and illustrated. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). titration is a method to determine the unknown concentration of a specific substance. the amount of added titrant is determined from its concentration and volume: to calculate concentration from titration, you can use the formula: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. C1v1 = c2v2 where c1 is the concentration of the titrant (the.
From printablekronloyaljn.z14.web.core.windows.net
How To Practice Concentration How To Calculate The Concentration From A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. C1v1 = c2v2 where c1 is the concentration of the titrant (the. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). titration is a method to determine the unknown concentration of a specific. How To Calculate The Concentration From A Titration.
From theedge.com.hk
Chemistry How To Titration The Edge How To Calculate The Concentration From A Titration the amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). to calculate concentration from titration, you. How To Calculate The Concentration From A Titration.
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog How To Calculate The Concentration From A Titration the amount of added titrant is determined from its concentration and volume: let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. . How To Calculate The Concentration From A Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate The Concentration From A Titration if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh).. How To Calculate The Concentration From A Titration.
From mungfali.com
Acid Base Titration Formula How To Calculate The Concentration From A Titration the process of calculating concentration from titration data is described and illustrated. the amount of added titrant is determined from its concentration and volume: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. . How To Calculate The Concentration From A Titration.
From www.youtube.com
The titration of 25 0 mL of an unknown concentration of H2SO4 solution How To Calculate The Concentration From A Titration to calculate concentration from titration, you can use the formula: the amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. if the concentration of the khp solution is known accurately and the titration of a naoh. How To Calculate The Concentration From A Titration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Calculate The Concentration From A Titration if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. the process of calculating concentration from titration data is described and illustrated. C1v1 = c2v2. How To Calculate The Concentration From A Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate The Concentration From A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. C1v1 = c2v2 where c1 is the concentration of the titrant (the. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. if the concentration of the khp solution is known accurately and the titration of. How To Calculate The Concentration From A Titration.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 How To Calculate The Concentration From A Titration let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the. How To Calculate The Concentration From A Titration.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution How To Calculate The Concentration From A Titration if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. the amount of added titrant is determined from its concentration and volume: to calculate concentration from titration, you can use the formula: let's assume you are titrating a strong acid (10 ml. How To Calculate The Concentration From A Titration.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog How To Calculate The Concentration From A Titration N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. to calculate concentration from titration, you can use the formula: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. How To Calculate The Concentration From A Titration.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d How To Calculate The Concentration From A Titration titration is a method to determine the unknown concentration of a specific substance. C1v1 = c2v2 where c1 is the concentration of the titrant (the. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. the process of calculating concentration from titration data is described and illustrated. to. How To Calculate The Concentration From A Titration.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Calculate The Concentration From A Titration the amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. to calculate concentration from titration, you can use the formula: if the. How To Calculate The Concentration From A Titration.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID3459790 How To Calculate The Concentration From A Titration to calculate concentration from titration, you can use the formula: the amount of added titrant is determined from its concentration and volume: titration is a method to determine the unknown concentration of a specific substance. the process of calculating concentration from titration data is described and illustrated. if the concentration of the khp solution is. How To Calculate The Concentration From A Titration.
From srkwxfutjcwku.blogspot.com
How To Find Initial Concentration From Titration Curve The initial How To Calculate The Concentration From A Titration the process of calculating concentration from titration data is described and illustrated. C1v1 = c2v2 where c1 is the concentration of the titrant (the. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a. How To Calculate The Concentration From A Titration.
From www.vrogue.co
Acid Base Titration Calculation Molar Concentration T vrogue.co How To Calculate The Concentration From A Titration C1v1 = c2v2 where c1 is the concentration of the titrant (the. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). titration is a method to determine the unknown concentration of a specific substance. N (mol) = c (mol /l) * v (l) and the amount of. How To Calculate The Concentration From A Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate The Concentration From A Titration to calculate concentration from titration, you can use the formula: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. the amount of added titrant is determined from its concentration and volume: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. C1v1 = c2v2. How To Calculate The Concentration From A Titration.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration How To Calculate The Concentration From A Titration let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. the amount of added titrant is determined from its concentration and volume: to calculate concentration from titration, you can use the formula: . How To Calculate The Concentration From A Titration.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog How To Calculate The Concentration From A Titration In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. the amount of added titrant is determined from its concentration and volume: C1v1 = c2v2 where c1 is the concentration of the titrant (the. the. How To Calculate The Concentration From A Titration.
From www.nagwa.com
Question Video Calculating the Mass of Solute in a Solution via How To Calculate The Concentration From A Titration N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). titration is a method to. How To Calculate The Concentration From A Titration.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog How To Calculate The Concentration From A Titration let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. to calculate concentration from titration, you can use the formula: C1v1 = c2v2. How To Calculate The Concentration From A Titration.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube How To Calculate The Concentration From A Titration the process of calculating concentration from titration data is described and illustrated. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. the amount of added titrant is determined from its concentration and volume: In a titration, 25.0 cm 3 of 0.100 mol/dm. How To Calculate The Concentration From A Titration.
From learningschoolmysticis9.z22.web.core.windows.net
How To Do Titrations In Chemistry How To Calculate The Concentration From A Titration to calculate concentration from titration, you can use the formula: the amount of added titrant is determined from its concentration and volume: In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. the process of calculating concentration from titration data is described and illustrated. let's assume you are titrating a strong acid. How To Calculate The Concentration From A Titration.
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog How To Calculate The Concentration From A Titration to calculate concentration from titration, you can use the formula: the process of calculating concentration from titration data is described and illustrated. C1v1 = c2v2 where c1 is the concentration of the titrant (the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. the amount of added titrant is determined from its. How To Calculate The Concentration From A Titration.
From www.youtube.com
Finding concentration of acid or base given amount needed to titrate How To Calculate The Concentration From A Titration C1v1 = c2v2 where c1 is the concentration of the titrant (the. to calculate concentration from titration, you can use the formula: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. the process of calculating concentration from titration data is described and illustrated. let's assume you are. How To Calculate The Concentration From A Titration.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry How To Calculate The Concentration From A Titration the process of calculating concentration from titration data is described and illustrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. C1v1 = c2v2 where c1 is the concentration of the titrant (the. let's. How To Calculate The Concentration From A Titration.
From www.chegg.com
Solved Using the titration data, determine the concentration How To Calculate The Concentration From A Titration the process of calculating concentration from titration data is described and illustrated. titration is a method to determine the unknown concentration of a specific substance. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is.. How To Calculate The Concentration From A Titration.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 How To Calculate The Concentration From A Titration N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. titration is a method to determine the unknown concentration of a specific substance. the. How To Calculate The Concentration From A Titration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Calculate The Concentration From A Titration C1v1 = c2v2 where c1 is the concentration of the titrant (the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. titration is a method to determine the unknown concentration of a specific substance. . How To Calculate The Concentration From A Titration.
From www.youtube.com
How to calculate molarity from titration data? Stock Solution vs How To Calculate The Concentration From A Titration let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). the process of calculating concentration from titration data is described and illustrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. if the concentration of the khp solution is known accurately and. How To Calculate The Concentration From A Titration.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube How To Calculate The Concentration From A Titration C1v1 = c2v2 where c1 is the concentration of the titrant (the. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. the process of calculating concentration from titration data is described and illustrated. the amount of added titrant is determined from its concentration and volume: In a titration,. How To Calculate The Concentration From A Titration.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube How To Calculate The Concentration From A Titration the amount of added titrant is determined from its concentration and volume: if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution is carried out. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. let's assume. How To Calculate The Concentration From A Titration.
From www.youtube.com
How to do a titration and calculate the concentration YouTube How To Calculate The Concentration From A Titration to calculate concentration from titration, you can use the formula: the amount of added titrant is determined from its concentration and volume: titration is a method to determine the unknown concentration of a specific substance. if the concentration of the khp solution is known accurately and the titration of a naoh solution with the khp solution. How To Calculate The Concentration From A Titration.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations How To Calculate The Concentration From A Titration let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. C1v1 = c2v2 where c1 is the concentration of the titrant (the. if the concentration of the khp solution. How To Calculate The Concentration From A Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation How To Calculate The Concentration From A Titration titration is a method to determine the unknown concentration of a specific substance. to calculate concentration from titration, you can use the formula: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the. C1v1 = c2v2 where c1 is the concentration of the titrant (the. the process of. How To Calculate The Concentration From A Titration.