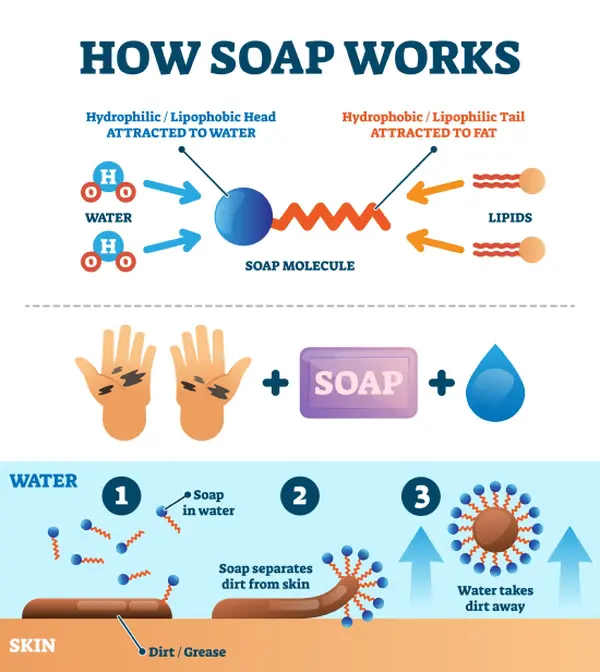
How Does Detergent Kill Bacteria . In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. Destroying the oil with a solvent like alcohol or kerosene will thus remove the associated germs. Micelles can also form around particles of dirt. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails first, squeezing their membrane envelopes and breaking them apart. Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the drain — along with its pathogenic prisoners — when you rinse the soap away with water.
from kidsclinic.sg
When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. Micelles can also form around particles of dirt. Destroying the oil with a solvent like alcohol or kerosene will thus remove the associated germs. Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the drain — along with its pathogenic prisoners — when you rinse the soap away with water. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails first, squeezing their membrane envelopes and breaking them apart. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin.
Is Antibacterial Handwash Better Than Regular Handwash? Kids Clinic
How Does Detergent Kill Bacteria In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. Micelles can also form around particles of dirt. Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. Destroying the oil with a solvent like alcohol or kerosene will thus remove the associated germs. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails first, squeezing their membrane envelopes and breaking them apart. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the drain — along with its pathogenic prisoners — when you rinse the soap away with water. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry.
From gioddtufy.blob.core.windows.net
Does Norwex Laundry Detergent Kill Bacteria at Isabelle Lance blog How Does Detergent Kill Bacteria Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. Destroying the oil with a solvent like alcohol or kerosene will thus remove the associated germs. Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the. How Does Detergent Kill Bacteria.
From fultondems.org
COVID19 Resources Fulton County Democrats How Does Detergent Kill Bacteria Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the drain — along with its pathogenic prisoners — when you rinse the soap away with water. Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into. How Does Detergent Kill Bacteria.
From cleanandtidyliving.com
What Temperature Kills Bacteria in a Washing Machine? How Does Detergent Kill Bacteria Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. When you. How Does Detergent Kill Bacteria.
From www.youtube.com
How Does Soap Kill Bacteria? Brut YouTube How Does Detergent Kill Bacteria Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the drain — along with its pathogenic. How Does Detergent Kill Bacteria.
From kidsclinic.sg
Is Antibacterial Handwash Better Than Regular Handwash? Kids Clinic How Does Detergent Kill Bacteria Micelles can also form around particles of dirt. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. When you wash your. How Does Detergent Kill Bacteria.
From www.rapidrestorationmn.com
Sanitizer vs Disinfectant & How to Kill Rapid Restoration How Does Detergent Kill Bacteria When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry. Destroying the oil with a solvent like alcohol or kerosene will thus remove the associated germs. Most of the gunk we want to wash off of our hands, whether. How Does Detergent Kill Bacteria.
From how2removestains.com
Do enzyme cleaners kill bacteria? [ Detailed Answer ] How Does Detergent Kill Bacteria When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails first, squeezing their membrane envelopes and breaking them apart. Most of the gunk we want to wash off of our hands, whether it be. How Does Detergent Kill Bacteria.
From newtondesk.com
Why Are Bubbles Formed In Soap Solution? Types of Soap How Does Detergent Kill Bacteria When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails first, squeezing their membrane envelopes and breaking them apart. Micelles can also form around particles of dirt. In tandem, some soap molecules disrupt the. How Does Detergent Kill Bacteria.
From sitn.hms.harvard.edu
Say Goodbye to Antibacterial Soaps Why the FDA is banning a household How Does Detergent Kill Bacteria In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails. How Does Detergent Kill Bacteria.
From www.walmart.com
Lysol Laundry Sanitizer, Sport, 41 oz, Eliminates Odors and Kills How Does Detergent Kill Bacteria In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails. How Does Detergent Kill Bacteria.
From scientificorigin.com
Human Cells Use Detergentlike Protein To Kill Bacteria How Does Detergent Kill Bacteria Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the drain — along with its pathogenic prisoners — when you rinse the soap away with water. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on. How Does Detergent Kill Bacteria.
From www.longislandlaundry.com
How to Sanitize Laundry in Cold Water? Long Island Laundry How Does Detergent Kill Bacteria Micelles can also form around particles of dirt. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner. How Does Detergent Kill Bacteria.
From www.thespruce.com
Does Your Hot Water Kill Bacteria? How Does Detergent Kill Bacteria Micelles can also form around particles of dirt. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails first, squeezing their membrane envelopes and breaking them apart. Rinsing then helps flush away any remaining. How Does Detergent Kill Bacteria.
From www.walmart.com
Lysol Laundry Sanitizer, Crisp Linen, 41 Oz, Eliminates Odors and Kills How Does Detergent Kill Bacteria Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and. How Does Detergent Kill Bacteria.
From www.killerinsideme.com
Can bacteria survive dish soap? How Does Detergent Kill Bacteria Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the drain — along with its pathogenic prisoners — when you rinse the soap away. How Does Detergent Kill Bacteria.
From www.ispot.tv
Clorox Fabric Sanitizer TV Commercial, 'Kills Bacteria Detergent Leaves How Does Detergent Kill Bacteria Micelles can also form around particles of dirt. Destroying the oil with a solvent like alcohol or kerosene will thus remove the associated germs. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails. How Does Detergent Kill Bacteria.
From cleanservant.com
Does Laundry Detergent Kill Germs Here is What You Should Know How Does Detergent Kill Bacteria Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards. How Does Detergent Kill Bacteria.
From www.youtube.com
Best Antifungal Laundry Detergent Kills Germs & Odor From Clothes How Does Detergent Kill Bacteria When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry. Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. Destroying the oil with a solvent like alcohol or. How Does Detergent Kill Bacteria.
From www.deal.com.lb
Dimex Laundry detergent Washing Powder 4 KG Kills Germs And Bacterias How Does Detergent Kill Bacteria Micelles can also form around particles of dirt. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard. How Does Detergent Kill Bacteria.
From www.activatedeco.com
Does Detergent Kill Bacteria? Insights and Tips How Does Detergent Kill Bacteria When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry. Micelles can also form around particles of dirt. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. When you wash. How Does Detergent Kill Bacteria.
From cleanservant.com
Does Laundry Detergent Kill Germs Here is What You Should Know How Does Detergent Kill Bacteria Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. Micelles can also form around particles of dirt. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. When you wash your hands with. How Does Detergent Kill Bacteria.
From www.pinterest.com
Get your sweat on. Kill the bacteria detergent leaves behind. Laundry How Does Detergent Kill Bacteria Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the drain — along with its pathogenic prisoners — when you rinse the soap away with water. Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into. How Does Detergent Kill Bacteria.
From www.longislandlaundry.com
Using Borax in Laundry How does it Work? Long Island Laundry How Does Detergent Kill Bacteria Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. Most of. How Does Detergent Kill Bacteria.
From examatri.blogspot.com
Laundry Detergent That Kills Bacteria Examatri Home Ideas How Does Detergent Kill Bacteria Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. Destroying the oil with a solvent like alcohol or kerosene will thus. How Does Detergent Kill Bacteria.
From richmondmom.com
Does Laundry Detergent Really Kill Bacteria? Richmond Mom How Does Detergent Kill Bacteria When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails first, squeezing their membrane envelopes and breaking them apart. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and. How Does Detergent Kill Bacteria.
From www.alamy.com
Antibacterial concept. Antiseptic spray in flask kills bacteria How Does Detergent Kill Bacteria Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. Rinsing then helps flush away any remaining detergent. How Does Detergent Kill Bacteria.
From www.dreamstime.com
Antibacterial Concept. Antiseptic Spray in Flask Kills Bacteria How Does Detergent Kill Bacteria Micelles can also form around particles of dirt. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails first, squeezing their membrane envelopes and breaking them apart. In tandem, some soap molecules disrupt the. How Does Detergent Kill Bacteria.
From examatri.blogspot.com
Laundry Detergent That Kills Bacteria Examatri Home Ideas How Does Detergent Kill Bacteria Micelles can also form around particles of dirt. In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. Bacteria or viruses are easily captured by micelles. How Does Detergent Kill Bacteria.
From laundrydetergentideas.com
Does Laundry Detergent Kill Bacteria on Clothes? How Does Detergent Kill Bacteria Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. When you wash your hands with soap, an army of detergent molecules surround the bacteria and viruses on your skin, and in an attempt to escape the surrounding water, they scurry towards and bombard them, tails first, squeezing their membrane. How Does Detergent Kill Bacteria.
From cleanservant.com
Does Laundry Detergent Kill Germs Here is What You Should Know How Does Detergent Kill Bacteria Micelles can also form around particles of dirt. In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. Most of the gunk we want to wash. How Does Detergent Kill Bacteria.
From laundrydetergentideas.com
Does Laundry Detergent Kill Germs? How Does Detergent Kill Bacteria Destroying the oil with a solvent like alcohol or kerosene will thus remove the associated germs. Micelles can also form around particles of dirt. Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to. How Does Detergent Kill Bacteria.
From globalhandwashing.org
How Washing Hands with Soap Destroys the Coronavirus The Global How Does Detergent Kill Bacteria Bacteria or viruses are easily captured by micelles because the outside of the micelle is hydrophilic, so it's easily swept off your hands and down the drain — along with its pathogenic prisoners — when you rinse the soap away with water. Most of the gunk we want to wash off of our hands, whether it be dirt or germs,. How Does Detergent Kill Bacteria.
From www.ispot.tv
Clorox Fabric Sanitizers TV Commercial, 'Kill Bacteria Detergent Can't How Does Detergent Kill Bacteria Agitation shakes dirt and bacteria loose from the fabric, while soaking allows the detergent to penetrate deeper into the fibers. In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. Most of the gunk we want to wash off of our hands, whether it be dirt. How Does Detergent Kill Bacteria.
From www.dreamstime.com
Dettol Antibacterial Surface Cleanser. Kills Bacteria and Viruses How Does Detergent Kill Bacteria Destroying the oil with a solvent like alcohol or kerosene will thus remove the associated germs. In tandem, some soap molecules disrupt the chemical bonds that allow bacteria, viruses and grime to stick to surfaces, lifting them off the skin. Essential proteins spill from the ruptured membranes into the surrounding water, killing the bacteria and rendering the viruses. Micelles can. How Does Detergent Kill Bacteria.
From www.walmart.com
Lysol Laundry Sanitizer, Crisp Linen, 90 oz, Eliminates Odors and Kills How Does Detergent Kill Bacteria Rinsing then helps flush away any remaining detergent and debris, leaving your clothes cleaner and less likely to have germs. Most of the gunk we want to wash off of our hands, whether it be dirt or germs, adheres to us thanks to the oils on our skin. Essential proteins spill from the ruptured membranes into the surrounding water, killing. How Does Detergent Kill Bacteria.