What Substances Are Soluble In Water . this set of rules is collectively known as solubility rules. They are specifically used to predict the solubility of ionic salts in water. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. not all ionic compounds are soluble in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Solubility rules can be used to predict whether a large number of ionic.
from www.numerade.com
a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. not all ionic compounds are soluble in water. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. They are specifically used to predict the solubility of ionic salts in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. Solubility rules can be used to predict whether a large number of ionic. this set of rules is collectively known as solubility rules.
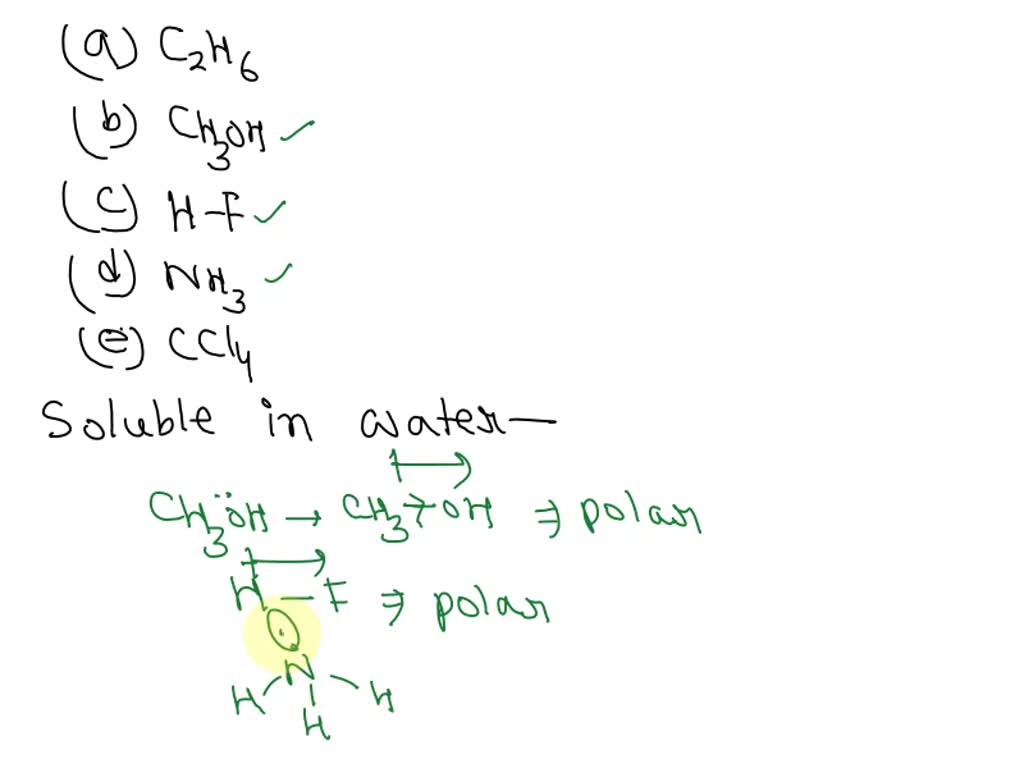
SOLVED Predict which of these covalent compounds is soluble in water (a) C2H6 (b) CH3OH (c) HF
What Substances Are Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. this set of rules is collectively known as solubility rules. Solubility rules can be used to predict whether a large number of ionic. not all ionic compounds are soluble in water. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. They are specifically used to predict the solubility of ionic salts in water. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in.
From www.ck12.org
Solubility CK12 Foundation What Substances Are Soluble In Water when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. this set of rules is collectively known as solubility rules. They are specifically used to predict the solubility of ionic salts in water. when some substances are dissolved in water, they undergo either a physical or a. What Substances Are Soluble In Water.
From www.numerade.com
SOLVED Predict which of these covalent compounds is soluble in water (a) C2H6 (b) CH3OH (c) HF What Substances Are Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. Solubility rules can be used to predict whether a large number of ionic. They are specifically used to. What Substances Are Soluble In Water.
From solutionpharmacy.in
Factors Influencing Solubility Solution Parmacy What Substances Are Soluble In Water Solubility rules can be used to predict whether a large number of ionic. not all ionic compounds are soluble in water. this set of rules is collectively known as solubility rules. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature.. What Substances Are Soluble In Water.
From brentongokemckay.blogspot.com
What Happens When an Ionic Compound Dissolves in Water What Substances Are Soluble In Water when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. this set of rules is collectively known as solubility rules. Solubility rules can be used to predict whether a large number of ionic. They are specifically used to predict the solubility of ionic salts in water. when. What Substances Are Soluble In Water.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous Solutions at Lois Witherspoon blog What Substances Are Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. They are specifically used to predict the solubility of ionic salts in water. Solubility rules can be used to predict whether a large number of ionic. this set of rules is collectively known as solubility rules.. What Substances Are Soluble In Water.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2869731 What Substances Are Soluble In Water They are specifically used to predict the solubility of ionic salts in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. a salt is soluble if it. What Substances Are Soluble In Water.
From www.numerade.com
SOLVED Rank the following substances in order from most soluble in water to least soluble in What Substances Are Soluble In Water this set of rules is collectively known as solubility rules. not all ionic compounds are soluble in water. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. when some substances are dissolved in water, they undergo either a physical. What Substances Are Soluble In Water.
From www.chegg.com
Solved Solubility rules for ionic compounds in water are What Substances Are Soluble In Water this set of rules is collectively known as solubility rules. Solubility rules can be used to predict whether a large number of ionic. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. a salt is soluble if it dissolves in water to give a solution with. What Substances Are Soluble In Water.
From www.thoughtco.com
Dissociation Reaction Definition and Examples What Substances Are Soluble In Water Solubility rules can be used to predict whether a large number of ionic. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. this set of rules is collectively known as solubility rules. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble. What Substances Are Soluble In Water.
From gracelimlf.blogspot.com
LIM LAI FONG (D20102043844) Science Year 3 What Substances Are Soluble In Water when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. They are specifically used to predict the solubility of ionic salts in water. not all. What Substances Are Soluble In Water.
From www.youtube.com
L8 Common soluble and insoluble substances, objects that float or sink in water, checkpoint 3 What Substances Are Soluble In Water this set of rules is collectively known as solubility rules. They are specifically used to predict the solubility of ionic salts in water. not all ionic compounds are soluble in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. rubidium formate, thallium formate, and silver perchlorate. What Substances Are Soluble In Water.
From www.chegg.com
Solved Rank These Compounds From Most Soluble In Water To... What Substances Are Soluble In Water not all ionic compounds are soluble in water. Solubility rules can be used to predict whether a large number of ionic. this set of rules is collectively known as solubility rules. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. a salt is soluble if. What Substances Are Soluble In Water.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous Solutions at Lois Witherspoon blog What Substances Are Soluble In Water not all ionic compounds are soluble in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. They are specifically used to predict the solubility of ionic salts in water. a salt is soluble if it dissolves in water to give a solution with a concentration. What Substances Are Soluble In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID1977818 What Substances Are Soluble In Water when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. not all ionic compounds are soluble in water. Solubility rules can. What Substances Are Soluble In Water.
From www.chemistrysteps.com
Solubility of Organic Compounds Chemistry Steps What Substances Are Soluble In Water They are specifically used to predict the solubility of ionic salts in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. not all ionic compounds are soluble in water. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000. What Substances Are Soluble In Water.
From www.youtube.com
water soluble substances and water insoluble substances YouTube What Substances Are Soluble In Water They are specifically used to predict the solubility of ionic salts in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. this set of rules is collectively known as solubility rules. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble. What Substances Are Soluble In Water.
From alfonsoewtfry.blogspot.com
Is Salt Soluble in Water AlfonsoewtFry What Substances Are Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. when some substances are dissolved in water, they undergo either a physical or a chemical. What Substances Are Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation ID5581895 What Substances Are Soluble In Water when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Solubility rules can be used to predict whether a large number of ionic. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room. What Substances Are Soluble In Water.
From www.numerade.com
SOLVED Arrange the organic compounds from most soluble in water t0 least soluble in water Most What Substances Are Soluble In Water when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. Solubility rules can be used to predict whether a large number of ionic. They are specifically used to predict the solubility of ionic salts in water. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble. What Substances Are Soluble In Water.
From surfguppy.com
Solubility Surfguppy Chemistry made easy for visual learners What Substances Are Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. They are specifically used to predict the solubility of ionic salts in water. Solubility rules can be used. What Substances Are Soluble In Water.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize What Substances Are Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. not all ionic compounds are soluble in water. Solubility rules can be used to predict whether a large number of ionic. this set of rules is collectively known as solubility rules. when some substances. What Substances Are Soluble In Water.
From www.breakingatom.com
Solubility of Elements and Compounds What Substances Are Soluble In Water They are specifically used to predict the solubility of ionic salts in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room. What Substances Are Soluble In Water.
From www.myxxgirl.com
Solubility Vector Illustration Labeled Solute Solvent And Solution My XXX Hot Girl What Substances Are Soluble In Water not all ionic compounds are soluble in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. this set of rules is collectively known as solubility rules. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in.. What Substances Are Soluble In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson What Substances Are Soluble In Water They are specifically used to predict the solubility of ionic salts in water. Solubility rules can be used to predict whether a large number of ionic. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. a salt is soluble if it dissolves in water to give a. What Substances Are Soluble In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids What Substances Are Soluble In Water rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. not all ionic compounds are soluble in water. this set of rules is collectively known as solubility rules. when some substances are dissolved in water, they undergo either a physical or a chemical change. What Substances Are Soluble In Water.
From rayb78.github.io
Solubility In Water Chart What Substances Are Soluble In Water a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Solubility rules can be used to predict whether a large number of ionic. They are specifically used to predict the solubility of ionic salts in water. not all ionic compounds are soluble. What Substances Are Soluble In Water.
From www.vrogue.co
Solubility Chemistry In Hindi Solubility With Tempera vrogue.co What Substances Are Soluble In Water Solubility rules can be used to predict whether a large number of ionic. not all ionic compounds are soluble in water. They are specifically used to predict the solubility of ionic salts in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. a salt is soluble if. What Substances Are Soluble In Water.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous Solutions at Lois Witherspoon blog What Substances Are Soluble In Water when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Solubility rules can be used to predict whether a large number of ionic. . What Substances Are Soluble In Water.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free download ID1820946 What Substances Are Soluble In Water They are specifically used to predict the solubility of ionic salts in water. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. not all ionic compounds are soluble in water. this set of rules is collectively known as solubility rules. a salt is. What Substances Are Soluble In Water.
From exogctlqx.blob.core.windows.net
Examples Of Not Soluble In Water at Edward Brafford blog What Substances Are Soluble In Water when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. not all ionic compounds are soluble in water. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. this set of rules is collectively known as solubility rules.. What Substances Are Soluble In Water.
From www.alamy.com
Solution science experiment. Solubility of salt and sand in water. Homogeneous, heterogeneous What Substances Are Soluble In Water not all ionic compounds are soluble in water. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. They. What Substances Are Soluble In Water.
From www.flinnsci.ca
Solubility Rules Chart, Notebook Size, Pad of 30 Flinn Scientific What Substances Are Soluble In Water not all ionic compounds are soluble in water. this set of rules is collectively known as solubility rules. They are specifically used to predict the solubility of ionic salts in water. Solubility rules can be used to predict whether a large number of ionic. a salt is soluble if it dissolves in water to give a solution. What Substances Are Soluble In Water.
From cassiusfersgardner.blogspot.com
All of the Following Compounds Are Soluble in Water Except What Substances Are Soluble In Water Solubility rules can be used to predict whether a large number of ionic. when some substances are dissolved in water, they undergo either a physical or a chemical change that yields. this set of rules is collectively known as solubility rules. They are specifically used to predict the solubility of ionic salts in water. a salt is. What Substances Are Soluble In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids What Substances Are Soluble In Water Solubility rules can be used to predict whether a large number of ionic. rubidium formate, thallium formate, and silver perchlorate are 3 of the most highly soluble compounds, with over 5,000 grams of each dissolving. this set of rules is collectively known as solubility rules. a salt is soluble if it dissolves in water to give a. What Substances Are Soluble In Water.
From brainly.com
Which of the following substances are likely to be soluble in water? A) ZnS B) Au2(CO3)3 C What Substances Are Soluble In Water this set of rules is collectively known as solubility rules. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. They are specifically used to predict the solubility of ionic salts in water. not all ionic compounds are soluble in water.. What Substances Are Soluble In Water.