Sulfuric Acid Titration Equation . The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). If a third titration was required, average the two closest values. Use the values for the averaged total volume of naoh added and the. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. Average the values for the total volumes of naoh added. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and alkalis. The example below demonstrates the. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq).
from www.chegg.com
Average the values for the total volumes of naoh added. The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Use the values for the averaged total volume of naoh added and the. The example below demonstrates the. Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and alkalis. If a third titration was required, average the two closest values. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \:
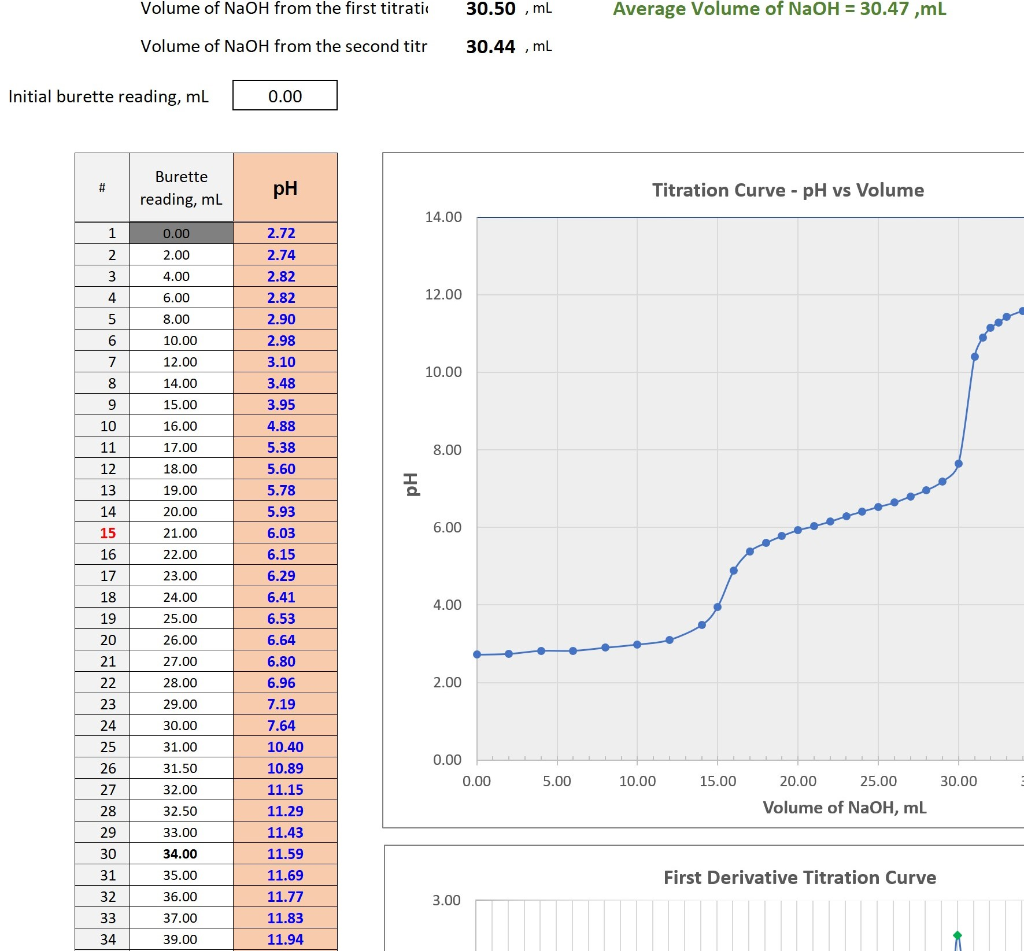
Titration of sulfuric acid Average Volume of NaOH =
Sulfuric Acid Titration Equation Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. The example below demonstrates the. If a third titration was required, average the two closest values. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and alkalis. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Average the values for the total volumes of naoh added. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Use the values for the averaged total volume of naoh added and the.
From www.numerade.com
SOLVED 'Reaction of sodium dichromate with potassium iodide in Sulfuric Acid Titration Equation Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Learn how to calculate the concentration of sulfuric acid solution by. Sulfuric Acid Titration Equation.
From www.chegg.com
Solved Be sure to answer all parts. The flask (below) Sulfuric Acid Titration Equation If a third titration was required, average the two closest values. The example below demonstrates the. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Use the values for the averaged total volume of naoh added and the. The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). In my latest chem lab. Sulfuric Acid Titration Equation.
From www.chegg.com
Solved 11. For the titration of sulfuric acid (H,S0.) with Sulfuric Acid Titration Equation If a third titration was required, average the two closest values. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). Average the values for the total volumes of naoh added. Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and alkalis. In a. Sulfuric Acid Titration Equation.
From ar.inspiredpencil.com
Sulfuric Acid And Sodium Hydroxide Sulfuric Acid Titration Equation The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). Use the values for the averaged total volume of naoh added and the. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. Learn how to use titration to measure the concentration or volume. Sulfuric Acid Titration Equation.
From www.numerade.com
SOLVED what happens when sodium hydroxide reacts with sulphuric acid Sulfuric Acid Titration Equation If a third titration was required, average the two closest values. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). Use the values for the averaged total volume of naoh added and. Sulfuric Acid Titration Equation.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Sulfuric Acid Titration Equation Average the values for the total volumes of naoh added. The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq).. Sulfuric Acid Titration Equation.
From polfido.weebly.com
Sulfuric acid formula balance chemical equations calculator polfido Sulfuric Acid Titration Equation In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Average the values for the total volumes of naoh added. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and. Sulfuric Acid Titration Equation.
From www.chegg.com
Titration of sulfuric acid Average Volume of NaOH = Sulfuric Acid Titration Equation Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. Use the values for the averaged total volume of naoh added and the. The example below demonstrates the. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: In my latest chem lab the objective was to create. Sulfuric Acid Titration Equation.
From www.youtube.com
How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric acid + Zinc) YouTube Sulfuric Acid Titration Equation The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. Use the values for the averaged total volume of naoh added and the. Sulfuric acid can be neutralised by. Sulfuric Acid Titration Equation.
From brainly.com
A solution of sulfuric acid is titrated with a solution of sodium Sulfuric Acid Titration Equation The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Use the values for the averaged total volume of naoh added and the. Learn how to use. Sulfuric Acid Titration Equation.
From syatillakmk.blogspot.com
SimplyChemistry TITRATION OF SULFURIC ACID WITH SODIUM HYDROXIDE Sulfuric Acid Titration Equation Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. The example below demonstrates the. Use the values for the averaged total volume of naoh added and the. If a third titration was required, average the two closest values. Learn how to use titration to measure the concentration or. Sulfuric Acid Titration Equation.
From www.numerade.com
SOLVED 2. (2 pts) Write the balanced chemical equation for the Sulfuric Acid Titration Equation Use the values for the averaged total volume of naoh added and the. Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and alkalis. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. In. Sulfuric Acid Titration Equation.
From www.numerade.com
SOLVED A titration is performed by reacting 18.57 mL of 0.353 M Sulfuric Acid Titration Equation In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). The. Sulfuric Acid Titration Equation.
From www.chegg.com
Solved 7. In a titration of sulfuric acid against sodium Sulfuric Acid Titration Equation The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). The example below demonstrates the. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. Average the values for the total volumes of naoh added. In my latest chem lab the objective was to. Sulfuric Acid Titration Equation.
From www.youtube.com
Balancing Chemical Equations. Part 2 Sulfuric Acid and Sodium Sulfuric Acid Titration Equation The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine. Sulfuric Acid Titration Equation.
From www.slideserve.com
PPT Titrations PowerPoint Presentation ID5572517 Sulfuric Acid Titration Equation Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and alkalis. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). Learn how to. Sulfuric Acid Titration Equation.
From studylib.net
2b. Ch 4 Barium hydroxide sulfuric acid titration Sulfuric Acid Titration Equation The example below demonstrates the. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide. Sulfuric Acid Titration Equation.
From byjus.com
36. Assertion Sodium Carbonate can be titrated against sulphuric acid Sulfuric Acid Titration Equation If a third titration was required, average the two closest values. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. Average the values for the total volumes of naoh added. The example below demonstrates the. Use the values for the averaged total volume of naoh added and the.. Sulfuric Acid Titration Equation.
From en.wikipedia.org
Sulfuric acid Wikipedia Sulfuric Acid Titration Equation Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. If a third titration was required, average the two closest values. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). The above equation works only for neutralizations in which there is a 1:1 ratio. Sulfuric Acid Titration Equation.
From www.bartleby.com
Answered Q3 In a titration of sulfuric acid… bartleby Sulfuric Acid Titration Equation Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. The example below demonstrates the. If a third titration was required, average the two closest values. The above equation works only for neutralizations in which. Sulfuric Acid Titration Equation.
From giomxxhve.blob.core.windows.net
Sulfuric Acid Titration Experiment at Frederick Duchesne blog Sulfuric Acid Titration Equation The example below demonstrates the. Average the values for the total volumes of naoh added. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and alkalis. Learn how to calculate the concentration of sulfuric. Sulfuric Acid Titration Equation.
From www.coursehero.com
[Solved] A 25.00 ml volume of sulfuric acid was titrated with a Sulfuric Acid Titration Equation In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and alkalis. Use the values for the averaged total volume of naoh added and the. If a third titration was required, average the two closest values. The above. Sulfuric Acid Titration Equation.
From www.academia.edu
(DOC) To determine the concentration of sulfuric acid by titration Sulfuric Acid Titration Equation Use the values for the averaged total volume of naoh added and the. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). The example below demonstrates the. Learn how to use titration to measure. Sulfuric Acid Titration Equation.
From byjus.com
What are the products obtained from the reaction of sodium chloride and Sulfuric Acid Titration Equation In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). Average the. Sulfuric Acid Titration Equation.
From www.numerade.com
SOLVED During a titration, 10.00 mL of sulphuric acid, H2SO4 is Sulfuric Acid Titration Equation Use the values for the averaged total volume of naoh added and the. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). In my latest chem lab the objective was to create a primary. Sulfuric Acid Titration Equation.
From www.numerade.com
SOLVED PART C CONCENTRATION OF SULFURIC ACID SOLUTION Minimize Sulfuric Acid Titration Equation In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). In a titration of sulfuric acid against sodium. Sulfuric Acid Titration Equation.
From mungfali.com
Acid Base Titration Calculation Sulfuric Acid Titration Equation If a third titration was required, average the two closest values. Average the values for the total volumes of naoh added. In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Use the values for the averaged total volume. Sulfuric Acid Titration Equation.
From www.chegg.com
Solved 1. The titration of 25.00 mL of a sulfuric acid Sulfuric Acid Titration Equation Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and alkalis. The example below demonstrates the. Use the values for the averaged total volume of naoh added and the. Average the values for the total volumes of naoh added. In a titration of sulfuric acid against sodium hydroxide,. Sulfuric Acid Titration Equation.
From www.youtube.com
Sodium Hydroxide + Sulfuric Acid Acid Base Neutralization Reaction Sulfuric Acid Titration Equation In a titration of sulfuric acid against sodium hydroxide, \(32.20 \: The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. If a third titration was required, average the two closest values. Average the values for the total volumes of naoh added. Learn how to calculate the concentration of sulfuric. Sulfuric Acid Titration Equation.
From www.chegg.com
Solved Be sure to answer all parts. The flask (below) Sulfuric Acid Titration Equation In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. Learn how to calculate the concentration of sulfuric acid solution by reaction with sodium hydroxide using a burette and phenolphthalein indicator. If a third titration was required, average the two closest values. Sulfuric acid can be neutralised. Sulfuric Acid Titration Equation.
From norah-chapter.blogspot.com
Sodium Bicarbonate And Sulfuric Acid Equation 20+ Pages Explanation Doc Sulfuric Acid Titration Equation In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). Learn how to use titration to measure the concentration or volume of a solution of sodium hydroxide (naoh) or other acids and. Sulfuric Acid Titration Equation.
From www.youtube.com
Acid Base Titration Sulfuric acid and Sodium hydroxide YouTube Sulfuric Acid Titration Equation Average the values for the total volumes of naoh added. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s). Sulfuric Acid Titration Equation.
From www.youtube.com
Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Sulfuric Acid Titration Equation If a third titration was required, average the two closest values. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. In a titration of sulfuric acid. Sulfuric Acid Titration Equation.
From www.numerade.com
SOLVED Q3 In a titration of sulfuric acid against sodium hydroxide Sulfuric Acid Titration Equation Use the values for the averaged total volume of naoh added and the. In my latest chem lab the objective was to create a primary standard of $\ce{naoh}$ and use it to determine the concentration of. The balanced ionic equation for this reaction is naoh(aq) + h2so4(aq) → na2so4(s) + h2o(l). The example below demonstrates the. Average the values for. Sulfuric Acid Titration Equation.
From www.chegg.com
Solved PART B Acid Base Titration Lab Report I Sulfuric Acid Titration Equation If a third titration was required, average the two closest values. Use the values for the averaged total volume of naoh added and the. Sulfuric acid can be neutralised by a strong base such as sodium hydroxide, naoh(aq). The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The example. Sulfuric Acid Titration Equation.