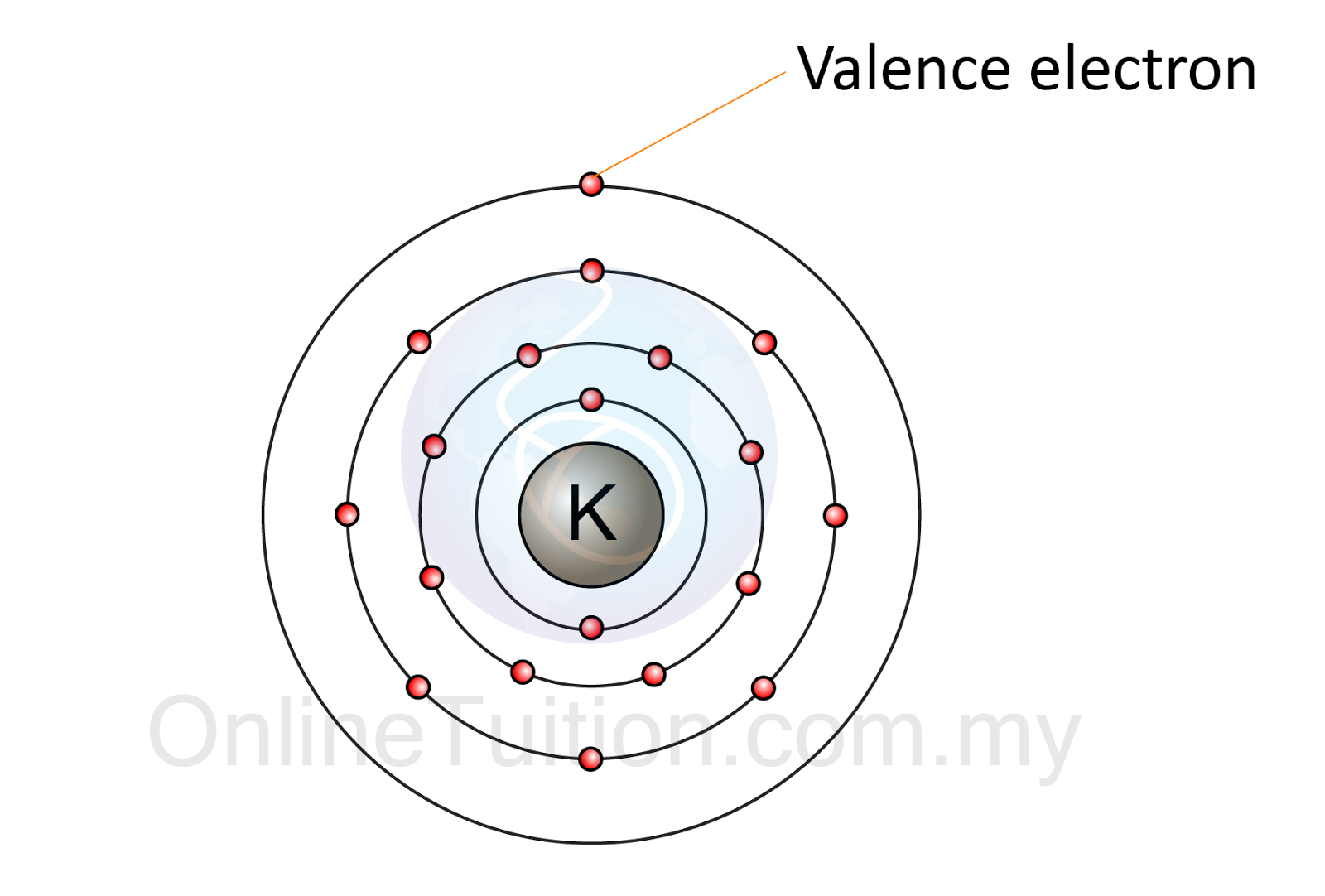
How Does Electron Arrangement Work . The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. No two electrons in an atom can have the same set of four quantum. The main idea behind electron arrangements is that electrons can only exist at certain energy levels. All other electrons are core electrons. The electron configuration can be represented using the electrons in boxes notation. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. The electron configuration states the arrangement of electrons in shells and subshells. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. Each box represents an atomic orbital. For example, the atomic number of sodium is 11. The electron arrangement of an atom can be worked out from its atomic number. Electrons are organized according to their energies into sets called shells. By understanding the energy levels of electrons in an atom, we can predict.
from spmchemistry.blog.onlinetuition.com.my
By understanding the energy levels of electrons in an atom, we can predict. The electron configuration states the arrangement of electrons in shells and subshells. The main idea behind electron arrangements is that electrons can only exist at certain energy levels. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: The electron arrangement of an atom can be worked out from its atomic number. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. No two electrons in an atom can have the same set of four quantum. All other electrons are core electrons. Electrons are organized according to their energies into sets called shells. For example, the atomic number of sodium is 11.
Electron Arrangement in Atom SPM Chemistry
How Does Electron Arrangement Work The electron configuration can be represented using the electrons in boxes notation. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: Each box represents an atomic orbital. The electron arrangement of an atom can be worked out from its atomic number. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. The main idea behind electron arrangements is that electrons can only exist at certain energy levels. By understanding the energy levels of electrons in an atom, we can predict. Electrons are organized according to their energies into sets called shells. All other electrons are core electrons. For example, the atomic number of sodium is 11. The electron configuration can be represented using the electrons in boxes notation. The electron configuration states the arrangement of electrons in shells and subshells. No two electrons in an atom can have the same set of four quantum. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and.
From users.highland.edu
Lewis Structures and Covalent Bonding How Does Electron Arrangement Work The electron configuration can be represented using the electrons in boxes notation. No two electrons in an atom can have the same set of four quantum. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. For example, the atomic number of sodium is 11. Orbital diagrams are pictorial representations. How Does Electron Arrangement Work.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics How Does Electron Arrangement Work Electrons are organized according to their energies into sets called shells. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. For example, the atomic number of sodium is 11. No two electrons in an atom can have the same set of four quantum. Orbital diagrams are pictorial representations of the electron configuration,. How Does Electron Arrangement Work.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell How Does Electron Arrangement Work The main idea behind electron arrangements is that electrons can only exist at certain energy levels. No two electrons in an atom can have the same set of four quantum. For example, the atomic number of sodium is 11. The electron configuration can be represented using the electrons in boxes notation. The electron arrangement of an atom can be worked. How Does Electron Arrangement Work.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho How Does Electron Arrangement Work The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. For example, the atomic number of sodium is 11. All other electrons are core electrons. The key to understanding electronic arrangement is summarized. How Does Electron Arrangement Work.
From courses.lumenlearning.com
Electrons Biology for Majors I How Does Electron Arrangement Work By understanding the energy levels of electrons in an atom, we can predict. Each box represents an atomic orbital. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. The electron configuration can be represented using the electrons in boxes notation. The electron configuration of an atom is the representation of the arrangement. How Does Electron Arrangement Work.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free How Does Electron Arrangement Work The electron configuration can be represented using the electrons in boxes notation. Electrons are organized according to their energies into sets called shells. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: The electron arrangement of an atom can. How Does Electron Arrangement Work.
From www.slideserve.com
PPT Electron Configurations PowerPoint Presentation, free download How Does Electron Arrangement Work No two electrons in an atom can have the same set of four quantum. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. For example, the atomic number of sodium is 11. Electrons are organized according to their energies into sets called shells. The key to understanding electronic arrangement. How Does Electron Arrangement Work.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica How Does Electron Arrangement Work Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. The electron configuration states the arrangement of electrons in shells and subshells. The main idea behind electron arrangements is that electrons can only exist at certain energy levels. All other electrons are core electrons. The electron arrangement of an atom can be worked. How Does Electron Arrangement Work.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram How Does Electron Arrangement Work The electron configuration states the arrangement of electrons in shells and subshells. No two electrons in an atom can have the same set of four quantum. The electron arrangement of an atom can be worked out from its atomic number. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: By understanding the energy levels of electrons. How Does Electron Arrangement Work.
From www.expii.com
Electrons — Structure & Properties Expii How Does Electron Arrangement Work By understanding the energy levels of electrons in an atom, we can predict. Each box represents an atomic orbital. No two electrons in an atom can have the same set of four quantum. All other electrons are core electrons. The electron configuration states the arrangement of electrons in shells and subshells. The electron configuration of an atom is the representation. How Does Electron Arrangement Work.
From app.pandai.org
Structure of the Atom How Does Electron Arrangement Work No two electrons in an atom can have the same set of four quantum. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. By understanding the energy levels of electrons in an atom, we can predict. The electron configuration states the arrangement of electrons in shells and subshells. Electrons are organized. How Does Electron Arrangement Work.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID2860057 How Does Electron Arrangement Work The electron configuration can be represented using the electrons in boxes notation. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. The electron arrangement of an atom can be worked out from its atomic number. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and. How Does Electron Arrangement Work.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table How Does Electron Arrangement Work For example, the atomic number of sodium is 11. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: The main idea behind electron arrangements is that electrons can only exist at certain energy levels. Electrons are organized according to their energies into sets called shells. Orbital diagrams are pictorial representations of the electron configuration, showing the. How Does Electron Arrangement Work.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy How Does Electron Arrangement Work The electron configuration can be represented using the electrons in boxes notation. The main idea behind electron arrangements is that electrons can only exist at certain energy levels. The electron arrangement of an atom can be worked out from its atomic number. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of.. How Does Electron Arrangement Work.
From www2.chemistry.msu.edu
Electron Configurations & The Periodic Table How Does Electron Arrangement Work Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. The electron configuration can be represented using the electrons in boxes notation. The electron configuration states the arrangement of electrons in shells and subshells. No two electrons in an atom can have the same set of four quantum. By understanding the energy levels. How Does Electron Arrangement Work.
From www.slideserve.com
PPT Electron Configuration PowerPoint Presentation, free download How Does Electron Arrangement Work The electron configuration states the arrangement of electrons in shells and subshells. The electron arrangement of an atom can be worked out from its atomic number. No two electrons in an atom can have the same set of four quantum. Electrons are organized according to their energies into sets called shells. All other electrons are core electrons. The electron configuration. How Does Electron Arrangement Work.
From www.tes.com
Electron Arrangement Part I OCR A Level Teaching Resources How Does Electron Arrangement Work The key to understanding electronic arrangement is summarized in the pauli exclusion principle: The electron arrangement of an atom can be worked out from its atomic number. Each box represents an atomic orbital. For example, the atomic number of sodium is 11. By understanding the energy levels of electrons in an atom, we can predict. The electron configuration can be. How Does Electron Arrangement Work.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table How Does Electron Arrangement Work The electron arrangement of an atom can be worked out from its atomic number. The main idea behind electron arrangements is that electrons can only exist at certain energy levels. Electrons are organized according to their energies into sets called shells. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. Each. How Does Electron Arrangement Work.
From www.youtube.com
Electron Arrangement YouTube How Does Electron Arrangement Work The electron configuration states the arrangement of electrons in shells and subshells. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: The main idea behind electron arrangements is that electrons can only exist at certain energy levels. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. Each. How Does Electron Arrangement Work.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind How Does Electron Arrangement Work The main idea behind electron arrangements is that electrons can only exist at certain energy levels. Each box represents an atomic orbital. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: For example, the atomic number of sodium is 11. The electron arrangement of an atom can be worked out from its atomic number. The electron. How Does Electron Arrangement Work.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science How Does Electron Arrangement Work Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. By understanding the energy levels of electrons in an atom, we can predict. Electrons are organized according to their energies into sets called shells. No. How Does Electron Arrangement Work.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes How Does Electron Arrangement Work The key to understanding electronic arrangement is summarized in the pauli exclusion principle: The electron configuration can be represented using the electrons in boxes notation. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the. How Does Electron Arrangement Work.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts How Does Electron Arrangement Work For example, the atomic number of sodium is 11. Each box represents an atomic orbital. Electrons are organized according to their energies into sets called shells. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. The electron configuration can be represented using the electrons in boxes notation. The electron arrangement of. How Does Electron Arrangement Work.
From gzscienceclassonline.weebly.com
1. Electron Configuration How Does Electron Arrangement Work The electron arrangement of an atom can be worked out from its atomic number. All other electrons are core electrons. By understanding the energy levels of electrons in an atom, we can predict. The main idea behind electron arrangements is that electrons can only exist at certain energy levels. Orbital diagrams are pictorial representations of the electron configuration, showing the. How Does Electron Arrangement Work.
From sciencenotes.org
List of Electron Configurations of Elements How Does Electron Arrangement Work By understanding the energy levels of electrons in an atom, we can predict. Electrons are organized according to their energies into sets called shells. Each box represents an atomic orbital. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. All other electrons are core electrons. The main idea behind electron arrangements. How Does Electron Arrangement Work.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry How Does Electron Arrangement Work Each box represents an atomic orbital. For example, the atomic number of sodium is 11. Electrons are organized according to their energies into sets called shells. The electron configuration states the arrangement of electrons in shells and subshells. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. The key to understanding. How Does Electron Arrangement Work.
From www.thoughtco.com
Electron Configuration Chart How Does Electron Arrangement Work The main idea behind electron arrangements is that electrons can only exist at certain energy levels. All other electrons are core electrons. The electron configuration can be represented using the electrons in boxes notation. The electron arrangement of an atom can be worked out from its atomic number. Orbital diagrams are pictorial representations of the electron configuration, showing the individual. How Does Electron Arrangement Work.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? How Does Electron Arrangement Work All other electrons are core electrons. The electron configuration can be represented using the electrons in boxes notation. The electron arrangement of an atom can be worked out from its atomic number. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. Generally the higher the energy of a shell,. How Does Electron Arrangement Work.
From studylib.net
Electron Arrangements How Does Electron Arrangement Work The electron configuration states the arrangement of electrons in shells and subshells. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. The main idea behind electron arrangements is that electrons can only. How Does Electron Arrangement Work.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science How Does Electron Arrangement Work The main idea behind electron arrangements is that electrons can only exist at certain energy levels. The electron arrangement of an atom can be worked out from its atomic number. Each box represents an atomic orbital. For example, the atomic number of sodium is 11. Electrons are organized according to their energies into sets called shells. Orbital diagrams are pictorial. How Does Electron Arrangement Work.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry How Does Electron Arrangement Work Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. The electron configuration states the arrangement of electrons in shells and subshells. The electron arrangement of an atom can be worked out from its atomic number. For example, the atomic number of sodium is 11. Generally the higher the energy of a. How Does Electron Arrangement Work.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps How Does Electron Arrangement Work All other electrons are core electrons. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: Each box represents an atomic orbital. By understanding the energy levels of electrons in an atom, we can predict. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. The main. How Does Electron Arrangement Work.
From www.youtube.com
Electron Arrangement in Atom Chemistry YouTube How Does Electron Arrangement Work Electrons are organized according to their energies into sets called shells. The key to understanding electronic arrangement is summarized in the pauli exclusion principle: Each box represents an atomic orbital. The main idea behind electron arrangements is that electrons can only exist at certain energy levels. No two electrons in an atom can have the same set of four quantum.. How Does Electron Arrangement Work.
From www.slideserve.com
PPT Regents Chemistry PowerPoint Presentation, free download ID333911 How Does Electron Arrangement Work The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and. By understanding the energy levels of electrons in an atom, we can predict. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of. The electron configuration can be represented using the electrons. How Does Electron Arrangement Work.
From chemistry.about.com
Basic Model of the Atom Atomic Theory How Does Electron Arrangement Work No two electrons in an atom can have the same set of four quantum. By understanding the energy levels of electrons in an atom, we can predict. Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. The electron configuration can be represented using the electrons in boxes notation. The electron configuration states. How Does Electron Arrangement Work.