Nitrogen Oxide Balanced Equation . To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Nitrous acid is an unstable. N2 + o2 = no2 is a synthesis reaction where one mole of dinitrogen [n 2] and two. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. All atoms are now balanced and the whole equation is fully balanced: When hydrogen gas reacts is combined with oxygen gas and the mixture. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write a balanced chemical equation for the reactions given below: Why there are two equations to express products of nitrogen dioxide and water reaction? We can explain it like this. 2 atoms in reagents and 2 atoms in products. N 2 + o 2 = 2 no Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Dinitrogen + dioxygen = nitrogen dioxide. To balance a chemical equation,.
from www.numerade.com
Nitrous acid is an unstable. N 2 + o 2 = 2 no When hydrogen gas reacts is combined with oxygen gas and the mixture. Why there are two equations to express products of nitrogen dioxide and water reaction? Use the calculator below to balance chemical equations and determine the type of reaction (instructions). N2 + o2 = no2 is a synthesis reaction where one mole of dinitrogen [n 2] and two. We can explain it like this. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Dinitrogen + dioxygen = nitrogen dioxide. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and.
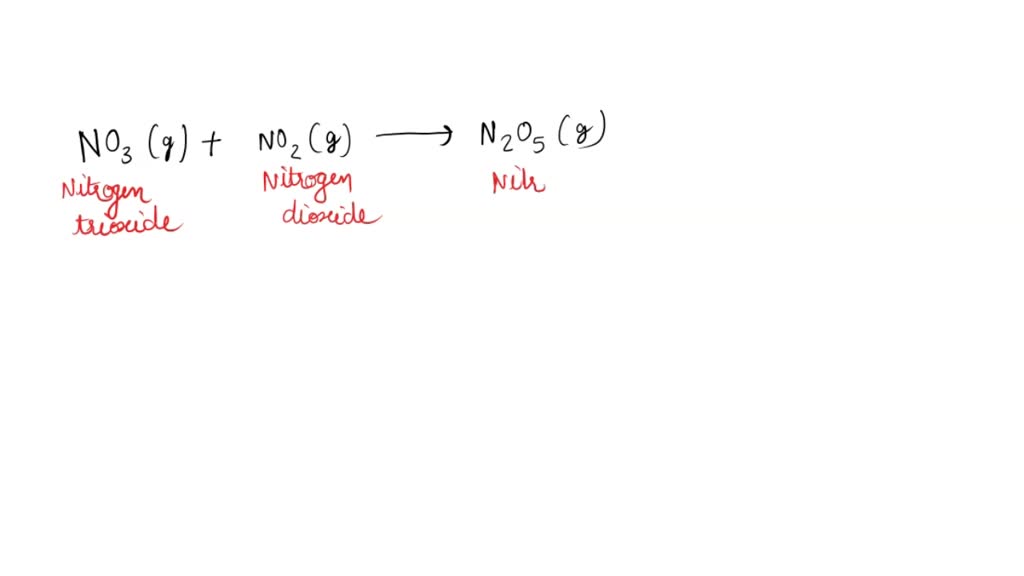
SOLVED Dinitrogen pentoxide gas is produced by the reaction of
Nitrogen Oxide Balanced Equation To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. When hydrogen gas reacts is combined with oxygen gas and the mixture. All atoms are now balanced and the whole equation is fully balanced: Why there are two equations to express products of nitrogen dioxide and water reaction? Use the calculator below to balance chemical equations and determine the type of reaction (instructions). N2 + o2 = no2 is a synthesis reaction where one mole of dinitrogen [n 2] and two. Nitrous acid is an unstable. 2 atoms in reagents and 2 atoms in products. Dinitrogen + dioxygen = nitrogen dioxide. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: To balance a chemical equation,. Write a balanced chemical equation for the reactions given below: N 2 + o 2 = 2 no We can explain it like this.
From byjus.com
Write the structures of oxides of nitogen also find the covalence of Nitrogen Oxide Balanced Equation We can explain it like this. Write a balanced chemical equation for the reactions given below: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Why there are two equations to express products of nitrogen dioxide and water reaction? All atoms are now balanced and the whole equation is fully balanced: Write a balanced. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVEDThe oxides of nitrogen are biologically reactive substances. NO Nitrogen Oxide Balanced Equation All atoms are now balanced and the whole equation is fully balanced: To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: 2 atoms in reagents and 2 atoms in products. Dinitrogen + dioxygen = nitrogen dioxide. Write a balanced chemical equation for the reactions given below: When hydrogen gas reacts. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVED Thank you for your help 1. Write the unbalanced, and balanced Nitrogen Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Dinitrogen + dioxygen = nitrogen dioxide. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Why there are two equations to express products of nitrogen dioxide and water reaction? To balance a chemical. Nitrogen Oxide Balanced Equation.
From www.alamy.com
Chemical formulas and molecule model of nitrogen oxide nitric oxide NO Nitrogen Oxide Balanced Equation All atoms are now balanced and the whole equation is fully balanced: Why there are two equations to express products of nitrogen dioxide and water reaction? Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Nitrous acid is an unstable. When hydrogen gas reacts is combined with oxygen gas and the mixture. Write a. Nitrogen Oxide Balanced Equation.
From mungfali.com
Balanced Equation For Nitrogen Oxide Nitrogen Oxide Balanced Equation To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). 2 atoms in reagents and 2 atoms in products. N 2 + o 2 = 2 no When hydrogen gas reacts is combined with oxygen gas. Nitrogen Oxide Balanced Equation.
From www.numerade.com
Write balanced chemical equations for each of the following reactions Nitrogen Oxide Balanced Equation Write a balanced chemical equation for the reactions given below: To balance a chemical equation,. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. N2 + o2 = no2 is a synthesis reaction where one mole of dinitrogen [n 2] and two. Why there are two equations to express products of nitrogen. Nitrogen Oxide Balanced Equation.
From mungfali.com
Balanced Equation For Nitrogen Oxide Nitrogen Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Why there are two equations to express products of nitrogen dioxide and water reaction? N2 + o2 = no2 is a synthesis reaction where one mole of dinitrogen [n 2] and two. N 2 + o 2 = 2 no When hydrogen. Nitrogen Oxide Balanced Equation.
From www.youtube.com
N2O=N2+O2 Balanced EquationNitrous oxide=Nitrogen+Oxygen Balanced Nitrogen Oxide Balanced Equation 2 atoms in reagents and 2 atoms in products. Why there are two equations to express products of nitrogen dioxide and water reaction? Write a balanced chemical equation for the reactions given below: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write a balanced equation for the decomposition of ammonium nitrate to form. Nitrogen Oxide Balanced Equation.
From brainly.in
Balance the equation nitrogen + oxygen > nitrous oxide Brainly.in Nitrogen Oxide Balanced Equation When hydrogen gas reacts is combined with oxygen gas and the mixture. All atoms are now balanced and the whole equation is fully balanced: Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Write a balanced chemical equation for the reactions given below: To balance a redox equation using the oxidation. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVED The mechanism for the reaction of nitrogen dioxide with carbon Nitrogen Oxide Balanced Equation Nitrous acid is an unstable. 2 atoms in reagents and 2 atoms in products. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Why there are two equations to express products of nitrogen dioxide and water reaction? When hydrogen gas reacts is combined with oxygen gas and the mixture. N 2. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite balanced net ionic equations for the following reactions Nitrogen Oxide Balanced Equation Nitrous acid is an unstable. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. N 2 + o 2 = 2 no When hydrogen gas reacts is combined with oxygen gas and the mixture. Why there are two equations to express products of nitrogen dioxide and water reaction? Write a balanced. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVED Nitrous acid (HNO2) disproportionates in acidic solutionto Nitrogen Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). N 2 + o 2 = 2 no We can explain it like this. Why there are two equations to express products of nitrogen dioxide and water. Nitrogen Oxide Balanced Equation.
From www.w3schools.blog
Oxides of nitrogen W3schools Nitrogen Oxide Balanced Equation N2 + o2 = no2 is a synthesis reaction where one mole of dinitrogen [n 2] and two. When hydrogen gas reacts is combined with oxygen gas and the mixture. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Nitrous acid is an unstable. 2 atoms in reagents and 2 atoms in products. Write. Nitrogen Oxide Balanced Equation.
From www.youtube.com
Writing balanced chemical equations from masses YouTube Nitrogen Oxide Balanced Equation We can explain it like this. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). 2 atoms in reagents and 2 atoms in products. Write a balanced chemical equation for the reactions given below: To balance a chemical equation,. N 2 + o 2 = 2 no Why there are two equations to express. Nitrogen Oxide Balanced Equation.
From www.vecteezy.com
Chemistry model molecule nitrogen oxide N2O scientific element formula Nitrogen Oxide Balanced Equation All atoms are now balanced and the whole equation is fully balanced: We can explain it like this. To balance a chemical equation,. When hydrogen gas reacts is combined with oxygen gas and the mixture. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. 2 atoms in reagents and 2 atoms. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite balanced chemical equations for each of the following Nitrogen Oxide Balanced Equation Dinitrogen + dioxygen = nitrogen dioxide. When hydrogen gas reacts is combined with oxygen gas and the mixture. N 2 + o 2 = 2 no Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Nitrous. Nitrogen Oxide Balanced Equation.
From www.youtube.com
molecular formula of an oxide of nitrogen from composition YouTube Nitrogen Oxide Balanced Equation Write a balanced chemical equation for the reactions given below: All atoms are now balanced and the whole equation is fully balanced: When hydrogen gas reacts is combined with oxygen gas and the mixture. We can explain it like this. 2 atoms in reagents and 2 atoms in products. Nitrous acid is an unstable. To balance a chemical equation,. Use. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced equation for each of the following processes Nitrogen Oxide Balanced Equation All atoms are now balanced and the whole equation is fully balanced: Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Why there are two equations to express products of nitrogen dioxide and water reaction? Nitrous acid is an unstable. Use the calculator below to balance chemical equations and determine the. Nitrogen Oxide Balanced Equation.
From hxeaqixkm.blob.core.windows.net
Nitrogen Oxide Ionic Formula at David Krehbiel blog Nitrogen Oxide Balanced Equation Why there are two equations to express products of nitrogen dioxide and water reaction? N 2 + o 2 = 2 no Use the calculator below to balance chemical equations and determine the type of reaction (instructions). All atoms are now balanced and the whole equation is fully balanced: We can explain it like this. To balance a redox equation. Nitrogen Oxide Balanced Equation.
From mungfali.com
Balanced Equation For Nitrogen Oxide Nitrogen Oxide Balanced Equation Why there are two equations to express products of nitrogen dioxide and water reaction? Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. We can explain it like this. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). When hydrogen gas reacts is combined with oxygen. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVED Dinitrogen pentoxide gas is produced by the reaction of Nitrogen Oxide Balanced Equation Write a balanced chemical equation for the reactions given below: N 2 + o 2 = 2 no Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Nitrous acid is an unstable. 2 atoms in reagents and 2 atoms in products. To balance a redox equation using the oxidation state method,. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced net ionic equation for each of the following Nitrogen Oxide Balanced Equation N2 + o2 = no2 is a synthesis reaction where one mole of dinitrogen [n 2] and two. When hydrogen gas reacts is combined with oxygen gas and the mixture. Write a balanced chemical equation for the reactions given below: To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Write. Nitrogen Oxide Balanced Equation.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas And Oxygen Gas Reaction Nitrogen Oxide Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: When hydrogen gas reacts is combined with oxygen gas and the mixture. N 2 + o 2 = 2 no To balance a chemical equation,. Write. Nitrogen Oxide Balanced Equation.
From www.chegg.com
Solved The chemistry of nitrogen oxides is very versatile. Nitrogen Oxide Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Why there are two equations to express products of nitrogen dioxide and water reaction? Dinitrogen + dioxygen = nitrogen dioxide. N2 + o2 = no2 is a synthesis reaction where one mole of dinitrogen [n 2] and two. When hydrogen gas reacts is combined with. Nitrogen Oxide Balanced Equation.
From mungfali.com
Balanced Equation For Nitrogen Oxide Nitrogen Oxide Balanced Equation When hydrogen gas reacts is combined with oxygen gas and the mixture. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write a balanced chemical equation for the reactions given below: All atoms are now balanced and the whole equation is fully balanced: N 2 + o 2 = 2 no Nitrous acid is. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVEDWhen nitrogen dioxide ( NO2 ) from car exhaust combines with Nitrogen Oxide Balanced Equation To balance a chemical equation,. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. When hydrogen gas reacts is combined with oxygen gas and the mixture. 2 atoms in reagents and 2 atoms in products. Why there are two equations to express products of nitrogen dioxide and water reaction? Nitrous acid. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVEDVarious nitrogen oxides, as well as sulfur oxides, contribute to Nitrogen Oxide Balanced Equation Why there are two equations to express products of nitrogen dioxide and water reaction? 2 atoms in reagents and 2 atoms in products. N 2 + o 2 = 2 no Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. All atoms are now balanced and the whole equation is fully. Nitrogen Oxide Balanced Equation.
From www.youtube.com
How to Balance N2 + O2 = NO2 (Nitrogen gas + Oxygen gas) YouTube Nitrogen Oxide Balanced Equation Nitrous acid is an unstable. We can explain it like this. When hydrogen gas reacts is combined with oxygen gas and the mixture. Dinitrogen + dioxygen = nitrogen dioxide. N 2 + o 2 = 2 no Why there are two equations to express products of nitrogen dioxide and water reaction? To balance a chemical equation,. Write a balanced equation. Nitrogen Oxide Balanced Equation.
From www.numerade.com
SOLVEDAmmonia reacts with oxygen to yield nitrogen and water. 4 NH3(g Nitrogen Oxide Balanced Equation Dinitrogen + dioxygen = nitrogen dioxide. Nitrous acid is an unstable. To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. When hydrogen gas reacts is combined with oxygen gas and the mixture. Write. Nitrogen Oxide Balanced Equation.
From www.researchgate.net
Simplified nitrogen oxides (NOx) reaction mechanism. Download Nitrogen Oxide Balanced Equation Nitrous acid is an unstable. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Why there are two equations to express products of nitrogen dioxide and water reaction? Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To balance a chemical equation,. Write a balanced chemical. Nitrogen Oxide Balanced Equation.
From www.chegg.com
Solved The reaction of carbon monoxide(g) with nitrogen Nitrogen Oxide Balanced Equation All atoms are now balanced and the whole equation is fully balanced: Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Why there are two equations to express products of nitrogen dioxide and water reaction? Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write a. Nitrogen Oxide Balanced Equation.
From hxelxlglm.blob.core.windows.net
Nitrous Oxide Balanced Equation at Donald Floyd blog Nitrogen Oxide Balanced Equation To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Dinitrogen + dioxygen = nitrogen dioxide. All atoms are now balanced and the whole equation is fully balanced: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write a balanced equation for the decomposition of. Nitrogen Oxide Balanced Equation.
From classnotes.org.in
Oxides of Nitrogen Chemistry, Class 12, The pBlock Elements Nitrogen Oxide Balanced Equation Nitrous acid is an unstable. Write a balanced chemical equation for the reactions given below: To balance a redox equation using the oxidation state method, we conceptually separate the overall reaction into two parts: Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. To balance a chemical equation,. Dinitrogen + dioxygen =. Nitrogen Oxide Balanced Equation.
From www.dreamstime.com
Nitrogen Dioxide NO2 Balanced Reaction 3D Illustration Stock Nitrogen Oxide Balanced Equation Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). We can explain it like this. Why there are two equations to express products of nitrogen dioxide and water reaction? When hydrogen gas reacts is combined with. Nitrogen Oxide Balanced Equation.
From hxelxlglm.blob.core.windows.net
Nitrous Oxide Balanced Equation at Donald Floyd blog Nitrogen Oxide Balanced Equation All atoms are now balanced and the whole equation is fully balanced: Why there are two equations to express products of nitrogen dioxide and water reaction? N2 + o2 = no2 is a synthesis reaction where one mole of dinitrogen [n 2] and two. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Write. Nitrogen Oxide Balanced Equation.