Graphite Equation . Graphite is a form of carbon with the molecular formula c. Graphite, mineral consisting of carbon. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. It has a hexagonal lattice of carbon atoms that. Its structure looks like this:
from www.bartleby.com
Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Graphite is a form of carbon with the molecular formula c. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms It has a hexagonal lattice of covalently bonded carbon atoms in layers. It has a hexagonal lattice of carbon atoms that. Graphite, mineral consisting of carbon. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. Its structure looks like this:
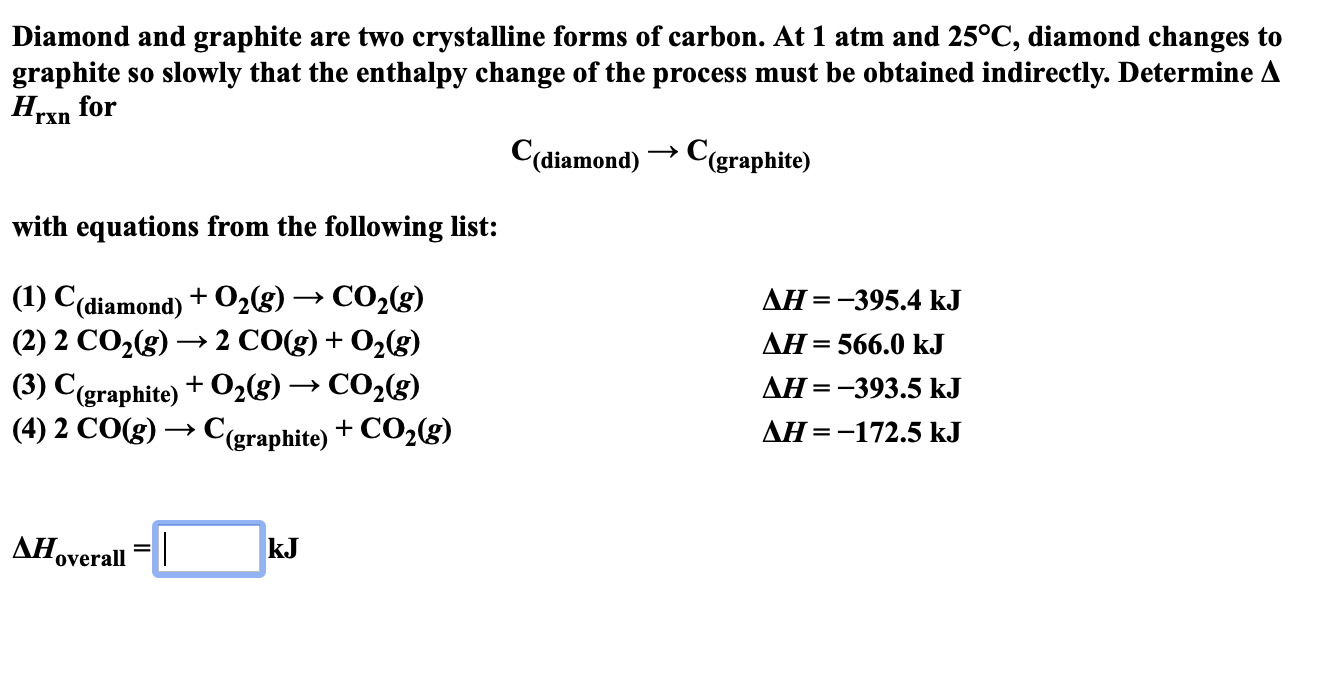
Answered Diamond and graphite are two… bartleby
Graphite Equation Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Graphite is a form of carbon with the molecular formula c. Its structure looks like this: It has a hexagonal lattice of carbon atoms that. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice.
From nanografi.com
What is the Difference Between Graphene Oxide and Reduced Graphene Graphite Equation Graphite, mineral consisting of carbon. Its structure looks like this: It has a hexagonal lattice of covalently bonded carbon atoms in layers. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. It has a hexagonal lattice of carbon atoms that. Graphite is simply an allotrope. Graphite Equation.
From www.researchgate.net
Graphite equation of states in cdirection and abplane. Color scheme Graphite Equation It has a hexagonal lattice of carbon atoms that. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as. Graphite Equation.
From www.youtube.com
What is the chemical formula of graphite? YouTube Graphite Equation Its structure looks like this: Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms It has a hexagonal lattice of carbon atoms that. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite. Graphite Equation.
From byjus.com
The heat of combustion of CH4(g),graphite and H2(g) are 20 kcal, 40 Graphite Equation Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms Graphite, mineral consisting of carbon. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Find the formula, molecular weight, structure, and thermochemistry data of. Graphite Equation.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID2755080 Graphite Equation Graphite, mineral consisting of carbon. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Graphite is a form of carbon with the molecular formula c. It has a hexagonal lattice of carbon atoms that.. Graphite Equation.
From socratic.org
What is the chemical formula of graphite? Socratic Graphite Equation Graphite, mineral consisting of carbon. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. Its structure looks like this: Graphite is a form of carbon with the molecular formula c. Graphite is simply an allotrope of carbon, and therefore. Graphite Equation.
From www.researchgate.net
1 (a) Graphene structure where the 2D hexagonal lattice of carbons is Graphite Equation Its structure looks like this: It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that. Graphite Equation.
From www.meritnation.com
Calculate the enthalpy change the transformation of C Graphite Equation Its structure looks like this: Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.”. Graphite Equation.
From brainly.in
(a ) define enthalpy of atomisation . the enthalpy of atomisation of Graphite Equation Graphite is a form of carbon with the molecular formula c. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms It has a hexagonal lattice of covalently bonded carbon atoms in layers. Find the formula, molecular. Graphite Equation.
From www.alamy.com
Graphite, crystal structure. Also known as pencil lead. Atoms from 3 Graphite Equation Graphite is a form of carbon with the molecular formula c. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite, mineral consisting of carbon. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in. Graphite Equation.
From www.mdpi.com
Materials Free FullText Investigation of Lithium Ion Diffusion of Graphite Equation Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms Graphite, mineral consisting of carbon. It has a hexagonal lattice. Graphite Equation.
From www.youtube.com
The enthalpy of combustion of methane, graphite and dihydrogen at 298K Graphite Equation Its structure looks like this: Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms It has a hexagonal. Graphite Equation.
From www.youtube.com
Allotropes of Carbon Graphite, Diamond, Graphene, & Fullerenes YouTube Graphite Equation Its structure looks like this: Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. It has a hexagonal lattice of covalently bonded carbon atoms in layers. It has a hexagonal lattice of carbon atoms that. Graphite is a form of carbon with the molecular formula. Graphite Equation.
From www.researchgate.net
Equilibrium partial pressures of gaseous carbon molecules over graphite Graphite Equation It has a hexagonal lattice of carbon atoms that. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite, mineral consisting of carbon. Graphite is simply an allotrope of carbon, and therefore only. Graphite Equation.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Graphite Equation Its structure looks like this: Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. It has a hexagonal lattice of carbon atoms that. Graphite, mineral consisting of carbon. Graphite is a form of carbon with the molecular formula c. It has a hexagonal lattice of. Graphite Equation.
From jinsuncarbon.com
What is the chemical formula of graphite? Jinsun Carbon Graphite Equation Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to. Graphite Equation.
From www.coursehero.com
[Solved] Suppose a powerful battery is connected between a pair of Graphite Equation Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. Its structure looks like this: It has a hexagonal lattice of covalently bonded carbon atoms in layers. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Graphite. Graphite Equation.
From www.nagwa.com
Question Video Identifying the Equation Used to Calculate the Graphite Equation Graphite is a form of carbon with the molecular formula c. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. It has a hexagonal lattice of carbon atoms that. Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus. Graphite Equation.
From www.toppr.com
0.562 g of graphite kept in a bomb calorimeter in excess of oxygen at Graphite Equation Graphite, mineral consisting of carbon. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite is a form of carbon with the molecular formula c. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Graphite has a greasy feel and leaves a black mark, thus the name from the. Graphite Equation.
From www.nagwa.com
Question Video Interpreting a Reaction Profile for the Conversion from Graphite Equation It has a hexagonal lattice of carbon atoms that. Graphite is a form of carbon with the molecular formula c. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms It has a hexagonal lattice of covalently. Graphite Equation.
From www.revisechemistry.uk
Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk Graphite Equation Its structure looks like this: Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms Graphite is simply an. Graphite Equation.
From www.numerade.com
SOLVED Graphite is burned in oxygen to give carbon monoxide and carbon Graphite Equation It has a hexagonal lattice of carbon atoms that. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. Graphite has a greasy feel and leaves a black. Graphite Equation.
From www.researchgate.net
The fugacity of CO in equilibrium with graphite (Equation 11) assuming Graphite Equation It has a hexagonal lattice of covalently bonded carbon atoms in layers. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. Graphite is a form of carbon with the molecular formula c. Its structure looks like this: Learn how the covalent bonding of carbon atoms in diamond and graphite affects their. Graphite Equation.
From tukioka-clinic.com
🎉 Structure and properties of graphite. Graphite A mineral with Graphite Equation Its structure looks like this: Graphite is a form of carbon with the molecular formula c. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms Graphite is simply an allotrope of carbon, and therefore only contains. Graphite Equation.
From www.researchgate.net
Schematic of graphite crystal structure (a) Graphite crystallite Graphite Equation Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Graphite is a form of carbon with the molecular formula c. Graphite has a greasy feel and leaves a. Graphite Equation.
From byjus.com
Describe the structure of Graphite. Graphite Equation Its structure looks like this: Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six. Graphite Equation.
From www.youtube.com
Thermodynamics ΔGº and K for the conversion of diamond to graphite Graphite Equation It has a hexagonal lattice of carbon atoms that. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Its structure looks like. Graphite Equation.
From www.takomabattery.com
Hard carbon anode for next generation lithium batteries theoretical Graphite Equation Graphite, mineral consisting of carbon. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Graphite has. Graphite Equation.
From glossary.periodni.com
Grafit Chemistry Dictionary & Glossary Graphite Equation Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Graphite is a form of carbon with the molecular formula c. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Find the formula, molecular weight, structure, and thermochemistry. Graphite Equation.
From byjus.com
Distinguish between graphite and diamond on the basis of their structures. Graphite Equation Graphite is a form of carbon with the molecular formula c. Graphite, mineral consisting of carbon. It has a hexagonal lattice of carbon atoms that. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and. Graphite Equation.
From www.researchgate.net
Charging and discharging of graphite. (a) Reaction equation for the Graphite Equation Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal lattice. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite is a form of carbon with the molecular formula c. Graphite has. Graphite Equation.
From solvedlib.com
260.562 g of graphite is placed in calorimeter with e… SolvedLib Graphite Equation Its structure looks like this: It has a hexagonal lattice of carbon atoms that. Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Find the formula, molecular weight, structure, and thermochemistry data of graphite, a form of carbon with hexagonal. Graphite Equation.
From byjus.com
51 For the allotropic change represented by the equation C(graphite Graphite Equation Graphite, mineral consisting of carbon. Its structure looks like this: Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. Graphite is a form of carbon with the molecular formula c. It has a hexagonal lattice of covalently bonded carbon atoms in layers. Graphite has a greasy feel and leaves a black mark,. Graphite Equation.
From www.researchgate.net
Schematic of the lithiumion battery with the graphite anode and LiCoO2 Graphite Equation Learn how the covalent bonding of carbon atoms in diamond and graphite affects their physical properties, such as melting point, hardness, conductivity and solubility. Graphite, mineral consisting of carbon. Its structure looks like this: Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula. It has a hexagonal lattice of covalently bonded carbon. Graphite Equation.
From www.bartleby.com
Answered Diamond and graphite are two… bartleby Graphite Equation Graphite, mineral consisting of carbon. Graphite has a greasy feel and leaves a black mark, thus the name from the greek verb graphein, “to write.” graphite has a layered structure that consists of rings of six carbon atoms Its structure looks like this: Graphite is simply an allotrope of carbon, and therefore only contains carbon atoms in its chemical formula.. Graphite Equation.