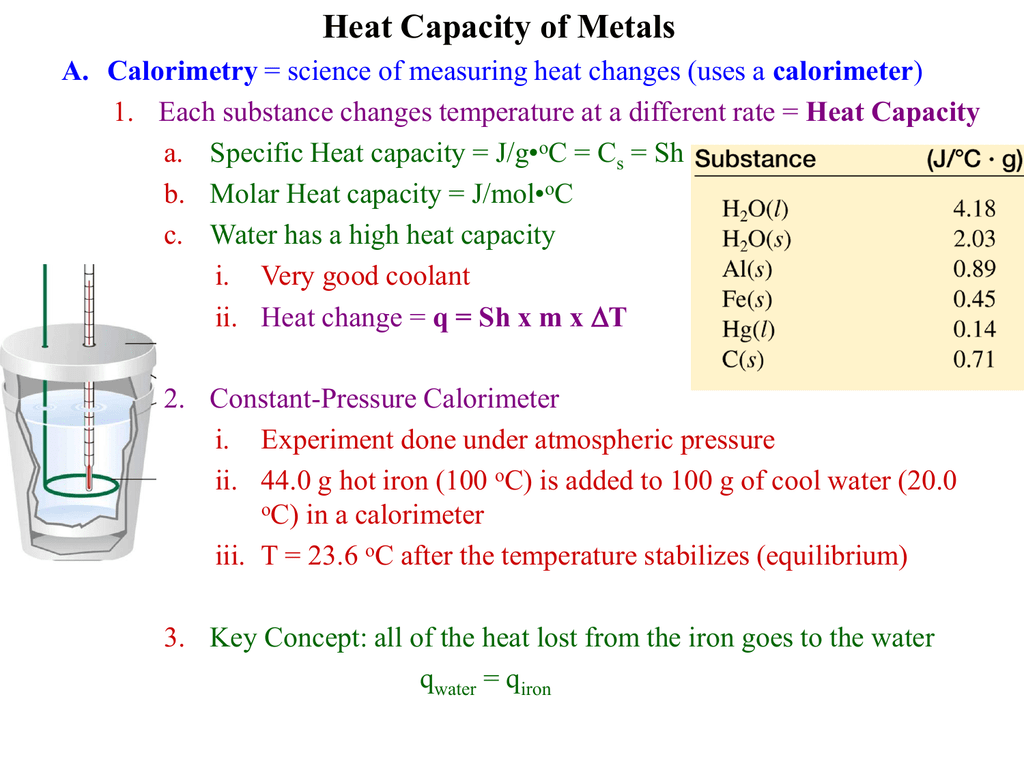
Lab Calorimetry And Specific Heat Student Guide . Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Then you will use this data and. • learn the experimental method of calorimetry. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. • determine the specific heat of. in this lab, you will do two classic calorimetry experiments: study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight.
from cechiggi.blob.core.windows.net
• learn the experimental method of calorimetry. Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. in this lab, you will do two classic calorimetry experiments: Then you will use this data and. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. • determine the specific heat of. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in.
Lab Calorimetry And Specific Heat at Alma Smith blog
Lab Calorimetry And Specific Heat Student Guide study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. • determine the specific heat of. Then you will use this data and. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. • learn the experimental method of calorimetry. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. in this lab, you will do two classic calorimetry experiments: Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals.
From www.chegg.com
LAB REPORT CALORIMETRY Specific heat of calorimeter Lab Calorimetry And Specific Heat Student Guide Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. Then you will use this data and. • learn the experimental method of calorimetry. study with quizlet and memorize flashcards containing terms like beryllium is a rare. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Chemistry b unit 2 lab calorimetry and specific heat student Lab Calorimetry And Specific Heat Student Guide • learn the experimental method of calorimetry. • determine the specific heat of. Then you will use this data and. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. first, the. Lab Calorimetry And Specific Heat Student Guide.
From www.scribd.com
Lab 07Specific Heat & Calorimetry PDF PDF Heat Temperature Lab Calorimetry And Specific Heat Student Guide • learn the experimental method of calorimetry. Then you will use this data and. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. in this lab, you will do two classic calorimetry experiments: you will use a calorimeter to collect data that. Lab Calorimetry And Specific Heat Student Guide.
From www.studocu.com
Copy of Lab 5 Calorimetry and Specific Heat Lab Calorimetry and Lab Calorimetry And Specific Heat Student Guide first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. in this lab, you will do two classic calorimetry experiments: • learn the experimental method of calorimetry. you will use a calorimeter to collect data that enables you to calculate the specific heat. Lab Calorimetry And Specific Heat Student Guide.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Lab Calorimetry And Specific Heat Student Guide • learn the experimental method of calorimetry. in this lab, you will do two classic calorimetry experiments: you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s),. Lab Calorimetry And Specific Heat Student Guide.
From ar.inspiredpencil.com
Specific Heat Lab Calorimeter Lab Calorimetry And Specific Heat Student Guide study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. Then you will use this data and. Measuring the latent heat of. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Lab Calorimetry And Specific Heat Student Guide Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals.. Lab Calorimetry And Specific Heat Student Guide.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity Lab Calorimetry And Specific Heat Student Guide Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. in this lab, you will do two classic calorimetry experiments: study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. • determine the specific heat of. you will use. Lab Calorimetry And Specific Heat Student Guide.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Lab Calorimetry And Specific Heat Student Guide in this lab, you will do two classic calorimetry experiments: Then you will use this data and. • learn the experimental method of calorimetry. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. study with quizlet and memorize flashcards containing terms like. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Lab calorimetry and specific heat test 2023 Studypool Lab Calorimetry And Specific Heat Student Guide • determine the specific heat of. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. in this lab, you will do two classic calorimetry experiments: the amount of heat absorbed. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Lab Calorimetry And Specific Heat Student Guide Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. • learn the experimental method of calorimetry. first, the specific heat (s) of a substance is the amount of heat. Lab Calorimetry And Specific Heat Student Guide.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student Lab Calorimetry And Specific Heat Student Guide • determine the specific heat of. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Purpose explore how the specific heat of a. Lab Calorimetry And Specific Heat Student Guide.
From www.scribd.com
Calorimetry Lab Heat Capacity Heat Lab Calorimetry And Specific Heat Student Guide Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Lab 2 introduction to calorimetry specific heat of a metal Lab Calorimetry And Specific Heat Student Guide Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. • learn the experimental method of calorimetry. • determine the specific heat of. first, the specific heat (s) of a substance is the amount of heat required. Lab Calorimetry And Specific Heat Student Guide.
From www.studocu.com
Exp 25 Calorimetry Lab guide Experiment 25 Calorimetry (Enthalpies Lab Calorimetry And Specific Heat Student Guide • determine the specific heat of. in this lab, you will do two classic calorimetry experiments: study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of. Lab Calorimetry And Specific Heat Student Guide.
From cechiggi.blob.core.windows.net
Lab Calorimetry And Specific Heat at Alma Smith blog Lab Calorimetry And Specific Heat Student Guide • determine the specific heat of. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. study with quizlet and memorize flashcards containing. Lab Calorimetry And Specific Heat Student Guide.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Lab Calorimetry And Specific Heat Student Guide you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Lab Calorimetry And Specific Heat Student Guide study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. • determine the specific heat of. Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. the amount of heat absorbed or released (q) by the object depends on its. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Lab calorimetry and specific heat test 2023 Studypool Lab Calorimetry And Specific Heat Student Guide Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. in this lab, you will do two classic calorimetry experiments: Measuring the latent heat of fusion of water,. Lab Calorimetry And Specific Heat Student Guide.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Lab Calorimetry And Specific Heat Student Guide • determine the specific heat of. Then you will use this data and. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. Lab Calorimetry And Specific Heat Student Guide.
From www.studocu.com
Calorimetry Part 1 Specific Heat Capacity Chem 200 Lab Report Lab Calorimetry And Specific Heat Student Guide Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. • determine the specific heat of. first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. study with quizlet and memorize flashcards containing terms like beryllium is a. Lab Calorimetry And Specific Heat Student Guide.
From www.qwivy.com
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Lab Calorimetry And Specific Heat Student Guide study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. • learn the experimental method of calorimetry. in this lab, you will do two classic. Lab Calorimetry And Specific Heat Student Guide.
From www.studocu.com
3210 07 04 student guide Copyright © Edgenuity Inc. Lab Calorimetry Lab Calorimetry And Specific Heat Student Guide first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Then you will use this data and. you will. Lab Calorimetry And Specific Heat Student Guide.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Lab Calorimetry And Specific Heat Student Guide you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. • determine the specific heat of. • learn the experimental method of calorimetry. in this. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Chemistry b unit 2 lab calorimetry and specific heat student Lab Calorimetry And Specific Heat Student Guide you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the. Lab Calorimetry And Specific Heat Student Guide.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Lab Calorimetry And Specific Heat Student Guide first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in. Lab Calorimetry And Specific Heat Student Guide.
From www.chegg.com
Laboratory 9 CALORIMETRY SPECIFIC HEAT OF A METAL Lab Calorimetry And Specific Heat Student Guide Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. • determine the specific heat of. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. in this lab, you will do two classic calorimetry experiments: first, the specific heat (s). Lab Calorimetry And Specific Heat Student Guide.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Lab Calorimetry And Specific Heat Student Guide • determine the specific heat of. • learn the experimental method of calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. Purpose explore how the specific heat of a substance can. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Lab Calorimetry And Specific Heat Student Guide in this lab, you will do two classic calorimetry experiments: • determine the specific heat of. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. • learn the experimental method of calorimetry. Measuring the latent heat of fusion of water, and measuring the. Lab Calorimetry And Specific Heat Student Guide.
From brainly.com
please i need it Lab Calorimetry and Specific Heat Lab Calorimetry And Specific Heat Student Guide the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. Then you will use this data and. • determine the specific heat of. Purpose explore how the specific heat. Lab Calorimetry And Specific Heat Student Guide.
From www.studocu.com
Lab 2 Introduction to Calorimetry Specific Heat of a Metal S23 Spring Lab Calorimetry And Specific Heat Student Guide • learn the experimental method of calorimetry. Then you will use this data and. Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. • determine the specific heat of. study with. Lab Calorimetry And Specific Heat Student Guide.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Lab Calorimetry And Specific Heat Student Guide you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. • determine the specific heat of. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. • learn the experimental method of calorimetry. first, the specific heat (s) of a substance is. Lab Calorimetry And Specific Heat Student Guide.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie Lab Calorimetry And Specific Heat Student Guide Purpose explore how the specific heat of a substance can be determined using a “coffee cup”. the amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. in this. Lab Calorimetry And Specific Heat Student Guide.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Lab Calorimetry And Specific Heat Student Guide you will use a calorimeter to collect data that enables you to calculate the specific heat of all four metals. in this lab, you will do two classic calorimetry experiments: first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. Then you will. Lab Calorimetry And Specific Heat Student Guide.
From studylib.net
Student instructions and lab report Lab Calorimetry And Specific Heat Student Guide first, the specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of the substance. • determine the specific heat of. in this lab, you will do two classic calorimetry experiments: Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two. study. Lab Calorimetry And Specific Heat Student Guide.