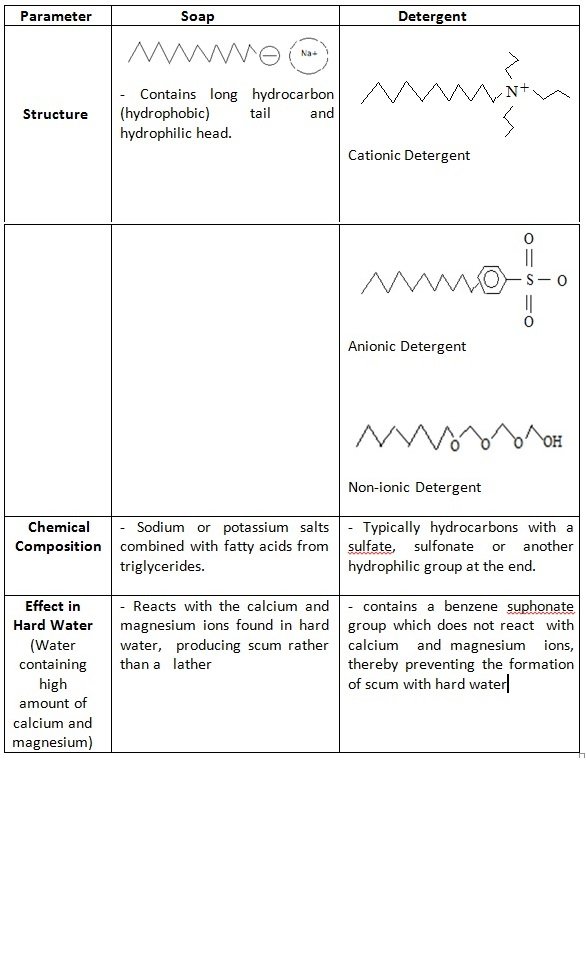
Hand Soap Chemical Properties . Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. The oldest amphiphilic cleaning agent known to humans is soap. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Have you ever seen a bead of water sitting on. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. While these products may seem simple, their effectiveness stems from the intricate chemistry behind their composition. The foundation of a good liquid hand soap lies in its ingredients. However, when soap and water are unavailable or when frequent handwashing compromises the skin’s natural barrier, hand sanitizers in foam, gel, or liquid form are recommended. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials. Understanding key ingredients in liquid hand soap. Soap is able to clean hands and dishes because of some pretty nifty chemistry.
from easychem.com.au
While these products may seem simple, their effectiveness stems from the intricate chemistry behind their composition. Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials. The oldest amphiphilic cleaning agent known to humans is soap. The foundation of a good liquid hand soap lies in its ingredients. Have you ever seen a bead of water sitting on. However, when soap and water are unavailable or when frequent handwashing compromises the skin’s natural barrier, hand sanitizers in foam, gel, or liquid form are recommended.
The Difference Between Soaps And Synthetic Detergents EasyChem Australia
Hand Soap Chemical Properties The foundation of a good liquid hand soap lies in its ingredients. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. Have you ever seen a bead of water sitting on. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. While these products may seem simple, their effectiveness stems from the intricate chemistry behind their composition. Understanding key ingredients in liquid hand soap. Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is able to clean hands and dishes because of some pretty nifty chemistry. However, when soap and water are unavailable or when frequent handwashing compromises the skin’s natural barrier, hand sanitizers in foam, gel, or liquid form are recommended. The foundation of a good liquid hand soap lies in its ingredients. The oldest amphiphilic cleaning agent known to humans is soap.
From ceh.org
8 NonToxic Hand Soaps Center for Environmental Health Hand Soap Chemical Properties The oldest amphiphilic cleaning agent known to humans is soap. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials. While these products may seem simple, their. Hand Soap Chemical Properties.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Hand Soap Chemical Properties Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. However, when soap and water. Hand Soap Chemical Properties.
From www.youtube.com
Chemistry 101 How does soap work? YouTube Hand Soap Chemical Properties Soap is able to clean hands and dishes because of some pretty nifty chemistry. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Have you ever seen a bead of water sitting on. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials.. Hand Soap Chemical Properties.
From www.scribd.com
Soap Soap Chemical Substances Hand Soap Chemical Properties The foundation of a good liquid hand soap lies in its ingredients. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Understanding key ingredients in liquid hand soap. Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. While these products. Hand Soap Chemical Properties.
From ar.inspiredpencil.com
Soap Molecule Structure Hand Soap Chemical Properties Soap is able to clean hands and dishes because of some pretty nifty chemistry. While these products may seem simple, their effectiveness stems from the intricate chemistry behind their composition. Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. The foundation of a good liquid hand soap lies in its. Hand Soap Chemical Properties.
From www.thoughtco.com
How Soap Works Hand Soap Chemical Properties However, when soap and water are unavailable or when frequent handwashing compromises the skin’s natural barrier, hand sanitizers in foam, gel, or liquid form are recommended. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials. Regular handwashing with soap and water is one of the most effective ways to prevent the spread. Hand Soap Chemical Properties.
From www.reddit.com
Chemistry of Soap versus Body Wash r/chemistry Hand Soap Chemical Properties Have you ever seen a bead of water sitting on. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Understanding key ingredients in liquid hand soap. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has. Hand Soap Chemical Properties.
From www.slideserve.com
PPT Preparation and Properties of a Soap PowerPoint Presentation Hand Soap Chemical Properties Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. The foundation of a good liquid hand soap lies in its ingredients. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Understanding key ingredients in liquid hand soap. Soap is. Hand Soap Chemical Properties.
From www.ncsshop.com.au
Advanced Chemicals Antibacterial Hand Soap White 5L NCS Cleaning Hand Soap Chemical Properties Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials. Understanding key ingredients in liquid hand soap. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The oldest amphiphilic cleaning agent known to humans is soap. While these products may seem simple,. Hand Soap Chemical Properties.
From thenerdyfarmwife.com
Soapmaking Oils Properties and Chart Hand Soap Chemical Properties While these products may seem simple, their effectiveness stems from the intricate chemistry behind their composition. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction. Hand Soap Chemical Properties.
From fr.dreamstime.com
Formule Générale De La Molécule De Savon Solide Et Liquide. Rcoona Hand Soap Chemical Properties Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The oldest amphiphilic cleaning agent known to humans is soap. Soap is able to clean hands and dishes because of some. Hand Soap Chemical Properties.
From www.clearvuehealth.com
Do you need to wash with soap? Visualized Science Hand Soap Chemical Properties However, when soap and water are unavailable or when frequent handwashing compromises the skin’s natural barrier, hand sanitizers in foam, gel, or liquid form are recommended. While these products may seem simple, their effectiveness stems from the intricate chemistry behind their composition. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials. Soaps. Hand Soap Chemical Properties.
From easychem.com.au
The Difference Between Soaps And Synthetic Detergents EasyChem Australia Hand Soap Chemical Properties Have you ever seen a bead of water sitting on. The oldest amphiphilic cleaning agent known to humans is soap. The foundation of a good liquid hand soap lies in its ingredients. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. While these products may seem simple, their effectiveness stems. Hand Soap Chemical Properties.
From www.webstaurantstore.com
Advantage Chemicals 1 Gallon Foaming Hand Soap Hand Soap Chemical Properties Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials. Have you ever seen. Hand Soap Chemical Properties.
From www.nvchemicals.com.au
AntiBacterial Liquid Hand Soap NV Chemicals Hand Soap Chemical Properties Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate. Hand Soap Chemical Properties.
From brainly.in
what is the difference between the molecules of soap and detergents Hand Soap Chemical Properties Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Understanding key ingredients in liquid hand soap. The foundation of a good liquid hand soap lies in its ingredients. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Each soap. Hand Soap Chemical Properties.
From studylib.net
LiquidHandSoapMSDS National Cleaning Supplies Hand Soap Chemical Properties Soap is able to clean hands and dishes because of some pretty nifty chemistry. The foundation of a good liquid hand soap lies in its ingredients. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. While these products may seem simple, their effectiveness. Hand Soap Chemical Properties.
From www.scribd.com
Soaps Soap Chemical Substances Hand Soap Chemical Properties The oldest amphiphilic cleaning agent known to humans is soap. Have you ever seen a bead of water sitting on. However, when soap and water are unavailable or when frequent handwashing compromises the skin’s natural barrier, hand sanitizers in foam, gel, or liquid form are recommended. Soap is able to clean hands and dishes because of some pretty nifty chemistry.. Hand Soap Chemical Properties.
From www.webstaurantstore.com
Advantage Chemicals 1 Gallon ReadytoUse Hand Soap 4/Case Hand Soap Chemical Properties The oldest amphiphilic cleaning agent known to humans is soap. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Have you ever seen a bead of water sitting on. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials. Regular handwashing with soap and water is. Hand Soap Chemical Properties.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID Hand Soap Chemical Properties In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. Understanding key ingredients in liquid hand soap. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soap molecules have on one end what’s known as a polar. Hand Soap Chemical Properties.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 Hand Soap Chemical Properties While these products may seem simple, their effectiveness stems from the intricate chemistry behind their composition. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and. Hand Soap Chemical Properties.
From www.gosupps.com
Sanit Silky Clean Antibacterial Hand Soap Refill 1 Gallon with Hand Soap Chemical Properties In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction. Hand Soap Chemical Properties.
From www.webstaurantstore.com
Advantage Chemicals 1 Gallon Hand Soap Hand Soap Chemical Properties Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Understanding key ingredients in liquid hand. Hand Soap Chemical Properties.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences Hand Soap Chemical Properties The foundation of a good liquid hand soap lies in its ingredients. However, when soap and water are unavailable or when frequent handwashing compromises the skin’s natural barrier, hand sanitizers in foam, gel, or liquid form are recommended. Have you ever seen a bead of water sitting on. Soap is able to clean hands and dishes because of some pretty. Hand Soap Chemical Properties.
From www.defeatdd.org
How does soap actually work? Hand Soap Chemical Properties Soap is able to clean hands and dishes because of some pretty nifty chemistry. Have you ever seen a bead of water sitting on. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Surfactants (yellow) cause water to lose surface tension, which is what keeps water separate from other materials.. Hand Soap Chemical Properties.
From www.webstaurantstore.com
Advantage Chemicals 1 Gallon Hand Soap Hand Soap Chemical Properties While these products may seem simple, their effectiveness stems from the intricate chemistry behind their composition. Have you ever seen a bead of water sitting on. The foundation of a good liquid hand soap lies in its ingredients. Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. Soap molecules have. Hand Soap Chemical Properties.
From umbelorganics.com
The Best NonToxic Hand Soap Umbel Organics Umbel Organics Hand Soap Chemical Properties Understanding key ingredients in liquid hand soap. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. Soaps are sodium or potassium fatty acids salts,. Hand Soap Chemical Properties.
From www.yumpu.com
Premium Line Hand Soap msds Perth Cleaning Supplies Hand Soap Chemical Properties Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. However, when soap and water are unavailable or when frequent handwashing compromises the skin’s natural barrier, hand sanitizers in foam, gel, or liquid form are recommended. The foundation of a good liquid hand soap lies in its ingredients. While these. Hand Soap Chemical Properties.
From www.slideshare.net
Chemistry of soaps Hand Soap Chemical Properties The oldest amphiphilic cleaning agent known to humans is soap. Understanding key ingredients in liquid hand soap. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The foundation of a good liquid hand soap lies in its ingredients. Soap molecules have on one end what’s known as a polar. Hand Soap Chemical Properties.
From parcoscientific.com
Properties of Soaps and Detergents Consumer Chemistry Chemistry Hand Soap Chemical Properties In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness. The oldest amphiphilic cleaning agent known to humans is soap. Have you ever seen a bead of water sitting on. Regular handwashing with soap and water is one of the most effective ways to. Hand Soap Chemical Properties.
From www.pinterest.fr
Hand washing with soap vector illustration. Educational explanation Hand Soap Chemical Properties Understanding key ingredients in liquid hand soap. Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction. Hand Soap Chemical Properties.
From www.slideshare.net
Chemistry of soaps Hand Soap Chemical Properties Soap is able to clean hands and dishes because of some pretty nifty chemistry. The oldest amphiphilic cleaning agent known to humans is soap. Regular handwashing with soap and water is one of the most effective ways to prevent the spread of illnesses. Have you ever seen a bead of water sitting on. While these products may seem simple, their. Hand Soap Chemical Properties.
From studylib.net
Preparation and Properties of Soap Hand Soap Chemical Properties Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is able to clean hands and dishes because of some pretty nifty chemistry. In this exploration of the chemistry of soap and detergents, we will uncover the science that makes these cleaning agents essential for maintaining hygiene and cleanliness.. Hand Soap Chemical Properties.
From www.coursehero.com
[Solved] Draw the chemical structure of a soap and a detergent (choose Hand Soap Chemical Properties Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The foundation of a good liquid hand soap lies in its ingredients. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Regular handwashing with soap and water is one of the most effective ways. Hand Soap Chemical Properties.
From www.slideserve.com
PPT Preparation and Properties of a Soap PowerPoint Presentation Hand Soap Chemical Properties Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. However, when soap and water are unavailable or when frequent handwashing compromises the skin’s natural barrier, hand sanitizers in foam, gel, or liquid form are recommended. Soap molecules have on one end what’s known as a polar salt, which is. Hand Soap Chemical Properties.