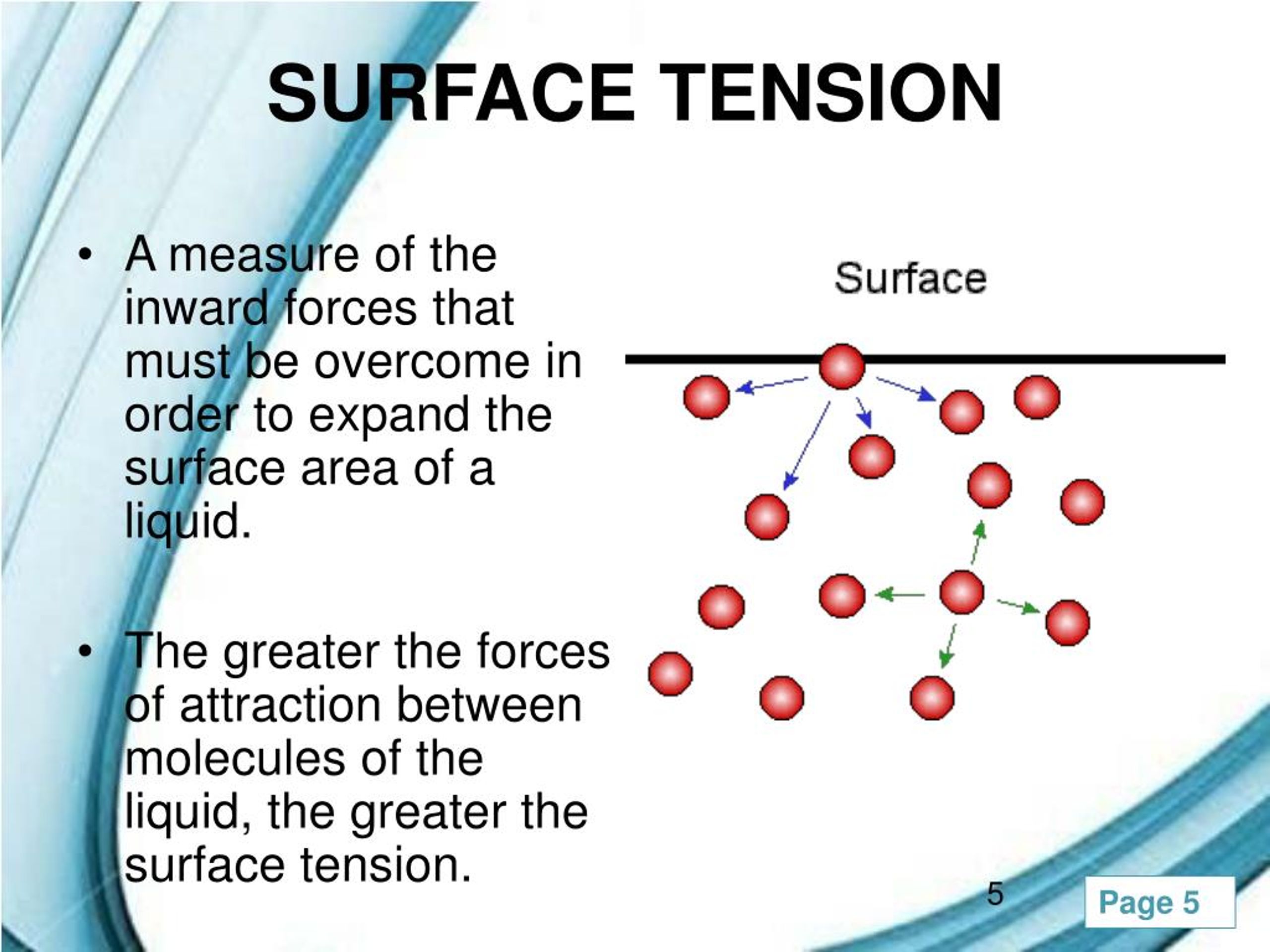
Liquids With High Surface Tension Are . Since these intermolecular forces vary. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between liquid molecules are responsible for the. An increase in temperature lowers. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. As a result of this high surface tension, the surface of water.
from www.slideserve.com
“surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. As a result of this high surface tension, the surface of water. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. An increase in temperature lowers. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between liquid molecules are responsible for the.
PPT Properties of Liquids and Solids PowerPoint Presentation, free
Liquids With High Surface Tension Are Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Since these intermolecular forces vary. The cohesive forces between liquid molecules are responsible for the. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. As a result of this high surface tension, the surface of water. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. An increase in temperature lowers.
From www.youtube.com
Surface Tension of Water Explained YouTube Liquids With High Surface Tension Are “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. An increase in temperature. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Liquids With High Surface Tension Are As a result of this high surface tension, the surface of water. The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.. Liquids With High Surface Tension Are.
From www.researchgate.net
a) Surface tension of various common liquids in air are compared with Liquids With High Surface Tension Are Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. An increase in temperature lowers. The cohesive forces between liquid molecules are responsible for the. “surface tension. Liquids With High Surface Tension Are.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Liquids With High Surface Tension Are An increase in temperature lowers. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. The cohesive forces between liquid molecules are responsible for the. As a result of this high surface tension, the surface of water. Surface tension. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID9168408 Liquids With High Surface Tension Are Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface. Liquids With High Surface Tension Are.
From www.thoughtco.com
Liquid Definition and Examples (Chemistry) Liquids With High Surface Tension Are The cohesive forces between liquid molecules are responsible for the. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Among common liquids, water exhibits. Liquids With High Surface Tension Are.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Liquids With High Surface Tension Are “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules are responsible for. Liquids With High Surface Tension Are.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Liquids With High Surface Tension Are Since these intermolecular forces vary. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. As a result of this high. Liquids With High Surface Tension Are.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Liquids With High Surface Tension Are As a result of this high surface tension, the surface of water. The cohesive forces between liquid molecules are responsible for the. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. In comparison, organic liquids, such as benzene. Liquids With High Surface Tension Are.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Liquids With High Surface Tension Are Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive. Liquids With High Surface Tension Are.
From www.youtube.com
What is Surface Tension Liquid State Liquid Dynamics Surface Liquids With High Surface Tension Are Since these intermolecular forces vary. The cohesive forces between liquid molecules are responsible for the. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer,. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 Liquids With High Surface Tension Are Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. As a result of this high surface tension, the. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free Liquids With High Surface Tension Are Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Since these intermolecular forces. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Chapter 12 Liquids and Solids PowerPoint Presentation, free Liquids With High Surface Tension Are An increase in temperature lowers. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. The cohesive forces between liquid molecules are responsible for the. Since these intermolecular forces vary. As a. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Chapter 15 Gases, Liquids, and Solids PowerPoint Presentation Liquids With High Surface Tension Are Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. An increase in temperature lowers. The cohesive forces between liquid molecules are responsible for the. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends. Liquids With High Surface Tension Are.
From users.highland.edu
Unique Properties of Liquids Liquids With High Surface Tension Are As a result of this high surface tension, the surface of water. An increase in temperature lowers. Since these intermolecular forces vary. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. In comparison, organic liquids, such as benzene. Liquids With High Surface Tension Are.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co Liquids With High Surface Tension Are Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Liquids With High Surface Tension Are An increase in temperature lowers. The cohesive forces between liquid molecules are responsible for the. Since these intermolecular forces vary. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the. Liquids With High Surface Tension Are.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Liquids With High Surface Tension Are Since these intermolecular forces vary. The cohesive forces between liquid molecules are responsible for the. As a result of this high surface tension, the surface of water. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury. Liquids With High Surface Tension Are.
From www.thoughtco.com
Surface Tension Definition in Chemistry Liquids With High Surface Tension Are Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. As a result of this high surface tension, the surface of water. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Among common liquids, water exhibits a distinctly high surface tension. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Properties of Liquids and Solids PowerPoint Presentation, free Liquids With High Surface Tension Are Since these intermolecular forces vary. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface tension, the surface of water. The cohesive forces between liquid molecules are responsible for the. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 13 Surfactants PowerPoint Presentation Liquids With High Surface Tension Are The cohesive forces between liquid molecules are responsible for the. An increase in temperature lowers. Since these intermolecular forces vary. As a result of this high surface tension, the surface of water. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to. Liquids With High Surface Tension Are.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Liquids With High Surface Tension Are The cohesive forces between liquid molecules are responsible for the. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. In comparison, organic liquids, such as benzene and alcohols, have lower surface. Liquids With High Surface Tension Are.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry Liquids With High Surface Tension Are An increase in temperature lowers. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. In comparison, organic liquids, such as benzene and alcohols, have lower surface. Liquids With High Surface Tension Are.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b Liquids With High Surface Tension Are Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. As a result of this high surface tension, the surface of water. Among common liquids,. Liquids With High Surface Tension Are.
From slideplayer.com
Chapter 15 “Water and Aqueous Systems” ppt download Liquids With High Surface Tension Are Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between. Liquids With High Surface Tension Are.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Liquids With High Surface Tension Are Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Liquids With High Surface Tension Are The cohesive forces between liquid molecules are responsible for the. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. As a result of this high surface tension, the surface of water. In comparison,. Liquids With High Surface Tension Are.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Liquids With High Surface Tension Are The cohesive forces between liquid molecules are responsible for the. As a result of this high surface tension, the surface of water. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Since these intermolecular forces vary. An increase. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free Liquids With High Surface Tension Are In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. As a result of this high surface tension, the surface of water. An increase in temperature lowers. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. “surface tension is. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Chapter 5 Liquids and Solids PowerPoint Presentation, free Liquids With High Surface Tension Are Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. An increase in temperature lowers. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. Liquids With High Surface Tension Are.
From www.dataphysics-instruments.com
Interfacial and surface tension of liquids explained DataPhysics Liquids With High Surface Tension Are In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The cohesive forces between liquid molecules are responsible for the. An increase in temperature lowers. Since these intermolecular forces vary. As a. Liquids With High Surface Tension Are.
From chem.libretexts.org
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts Liquids With High Surface Tension Are The cohesive forces between liquid molecules are responsible for the. An increase in temperature lowers. Since these intermolecular forces vary. As a result of this high surface tension, the surface of water. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as benzene and. Liquids With High Surface Tension Are.
From www.youtube.com
Why are liquid drops spherical? Surface Tension YouTube Liquids With High Surface Tension Are As a result of this high surface tension, the surface of water. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules are responsible for the. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules.. Liquids With High Surface Tension Are.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Liquids With High Surface Tension Are An increase in temperature lowers. In comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a higher surface tension. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Since these intermolecular forces. Liquids With High Surface Tension Are.