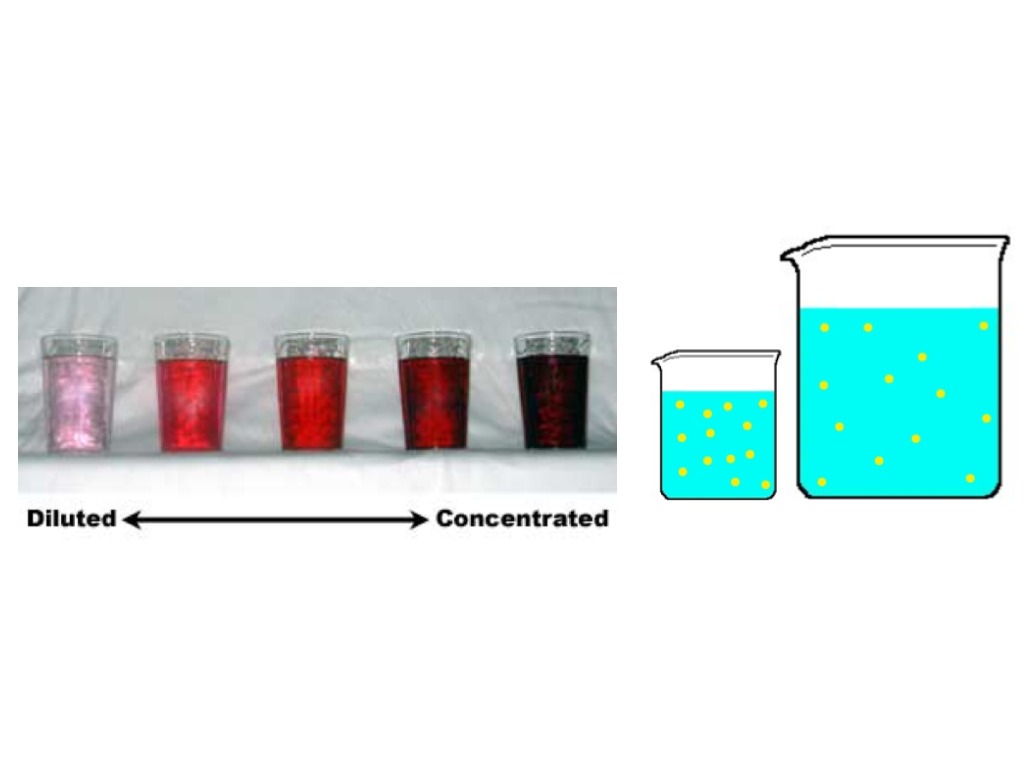
Diluted With Water To Make A Solution . You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. For example, if you have a glass. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Performing a dilution in chemistry usually means taking a small amount of a. The diluted liquid needs to be thoroughly. In dilution, we add more solvent to a solution of a higher concentration to. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). The new molarity can easily be calculated by. We can take advantage of dilution to make solutions of. You dilute the solution by adding enough water to make the solution volume \(500. You can easily calculate this value,. When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution.
from www.showme.com
You can easily calculate this value,. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. The new molarity can easily be calculated by. We can take advantage of dilution to make solutions of. Performing a dilution in chemistry usually means taking a small amount of a. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). You dilute the solution by adding enough water to make the solution volume \(500. In dilution, we add more solvent to a solution of a higher concentration to. For example, if you have a glass.
Concentration solution vs diluted solution Science ShowMe
Diluted With Water To Make A Solution When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution. In dilution, we add more solvent to a solution of a higher concentration to. You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. You dilute the solution by adding enough water to make the solution volume \(500. You can easily calculate this value,. For example, if you have a glass. The new molarity can easily be calculated by. The diluted liquid needs to be thoroughly. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. We can take advantage of dilution to make solutions of. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Performing a dilution in chemistry usually means taking a small amount of a. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution.
From www.slideserve.com
PPT Solution Concentration (Molarity) PowerPoint Presentation, free Diluted With Water To Make A Solution We can take advantage of dilution to make solutions of. The diluted liquid needs to be thoroughly. The new molarity can easily be calculated by. You can easily calculate this value,. In dilution, we add more solvent to a solution of a higher concentration to. Performing a dilution in chemistry usually means taking a small amount of a. Reconstitution is. Diluted With Water To Make A Solution.
From animalia-life.club
Solutions Examples Diluted With Water To Make A Solution You dilute the solution by adding enough water to make the solution volume \(500. Performing a dilution in chemistry usually means taking a small amount of a. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. The new molarity can easily be calculated by. The diluted liquid needs to be thoroughly. When. Diluted With Water To Make A Solution.
From www.youtube.com
Dilute and Concentrated Solution YouTube Diluted With Water To Make A Solution The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). In dilution, we add more solvent to a solution of a higher concentration to. The diluted liquid needs to be thoroughly. Performing a dilution in chemistry usually means taking a small amount of a. When we add water to a solution,. Diluted With Water To Make A Solution.
From byjus.com
50 ml of 2N hcl is diluted by 950 ml water.The ph of resul†an t Diluted With Water To Make A Solution You can easily calculate this value,. You dilute the solution by adding enough water to make the solution volume \(500. The new molarity can easily be calculated by. When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution. You can use this calculator to determine how much. Diluted With Water To Make A Solution.
From www.numerade.com
SOLVED Serial dilution is a common technique used in chemical analysis Diluted With Water To Make A Solution The new molarity can easily be calculated by. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. In dilution, we add more solvent to a solution of a higher concentration to. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). For example,. Diluted With Water To Make A Solution.
From slideplayer.com
Making a solution. ppt download Diluted With Water To Make A Solution Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a. Diluted With Water To Make A Solution.
From www.chegg.com
Solved Question 11 Of 13 How Many ML Of 0.300 M NaF Would... Diluted With Water To Make A Solution In dilution, we add more solvent to a solution of a higher concentration to. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). You dilute the solution by adding enough water to make the solution volume \(500. We can take advantage of dilution to make solutions of. You can easily. Diluted With Water To Make A Solution.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Diluted With Water To Make A Solution You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. We can take advantage of dilution to make solutions of. For example, if you have a glass. Dilution is the process of making a solution or scientific sample less concentrated. Diluted With Water To Make A Solution.
From www.chegg.com
Solved A 57.17g sample of Ba(OH)2 is dissolved in enough Diluted With Water To Make A Solution When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution. The diluted liquid needs to be thoroughly. You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm.. Diluted With Water To Make A Solution.
From sonia-has-mcneil.blogspot.com
Describe How Dilution Was Used to Prepare the Starch Solutions Sonia Diluted With Water To Make A Solution The new molarity can easily be calculated by. You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. You dilute the solution by. Diluted With Water To Make A Solution.
From brainly.in
calculate the molarity of NaOH in the solution prepared by dissolving Diluted With Water To Make A Solution The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). The diluted liquid needs to be thoroughly. The new molarity can easily be calculated by. For example, if you have a glass. You can easily calculate this value,. You dilute the solution by adding enough water to make the solution volume. Diluted With Water To Make A Solution.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Diluted With Water To Make A Solution For example, if you have a glass. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. We can take advantage of dilution to make solutions of. In dilution, we add more solvent to a solution of a higher concentration to. The dilution ratio is the ratio of the solute (the substance to. Diluted With Water To Make A Solution.
From www.sciencekiddo.com
Red Cabbage pH Indicator Kitchen Chemistry for Kids Science Kiddo Diluted With Water To Make A Solution For example, if you have a glass. When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution. You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm.. Diluted With Water To Make A Solution.
From www.showme.com
Concentration solution vs diluted solution Science ShowMe Diluted With Water To Make A Solution Performing a dilution in chemistry usually means taking a small amount of a. You can easily calculate this value,. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). We can take advantage of dilution to make solutions of. You dilute the solution by adding enough water to make the solution. Diluted With Water To Make A Solution.
From www.slideshare.net
Chapter 16 solutions Diluted With Water To Make A Solution The diluted liquid needs to be thoroughly. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). The new molarity can easily be calculated by. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. You can easily calculate this value,. For example, if. Diluted With Water To Make A Solution.
From www.chegg.com
Solved Question During The First Week Of This Project, Y... Diluted With Water To Make A Solution The diluted liquid needs to be thoroughly. You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution.. Diluted With Water To Make A Solution.
From www.numerade.com
SOLVED 72.84 g sample of Ba(OH)2 is dissolved in enough water to make Diluted With Water To Make A Solution Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. The diluted liquid needs to be thoroughly. Performing a dilution in chemistry usually means taking a small amount of a. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. You can use this calculator to. Diluted With Water To Make A Solution.
From www.slideshare.net
Water and solutions Diluted With Water To Make A Solution The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). The new molarity can easily be calculated by. We can take advantage of dilution to make solutions of. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. In dilution, we add more solvent. Diluted With Water To Make A Solution.
From saylordotorg.github.io
Solution Concentrations Diluted With Water To Make A Solution You dilute the solution by adding enough water to make the solution volume \(500. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. For example, if you have a glass. The diluted liquid needs to. Diluted With Water To Make A Solution.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Diluted With Water To Make A Solution For example, if you have a glass. You dilute the solution by adding enough water to make the solution volume \(500. The new molarity can easily be calculated by. Performing a dilution in chemistry usually means taking a small amount of a. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. You. Diluted With Water To Make A Solution.
From socratic.org
How many milliliters of 12.0 M HCl(aq) must be diluted with water to Diluted With Water To Make A Solution The new molarity can easily be calculated by. Performing a dilution in chemistry usually means taking a small amount of a. Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. You dilute the solution by adding enough water to make the solution volume \(500. The dilution ratio is the ratio of the. Diluted With Water To Make A Solution.
From byjus.com
The correct way of making a solution of acid in water is to Diluted With Water To Make A Solution You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. For example, if you have a glass. The diluted liquid needs to be. Diluted With Water To Make A Solution.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Diluted With Water To Make A Solution You can easily calculate this value,. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. Reconstitution is the process of. Diluted With Water To Make A Solution.
From chem.libretexts.org
4.5 Concentration of Solutions Chemistry LibreTexts Diluted With Water To Make A Solution The new molarity can easily be calculated by. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. You can easily calculate this value,. In dilution, we add more solvent to a solution of a higher concentration to. We can take advantage of dilution to make solutions of. The diluted liquid needs to. Diluted With Water To Make A Solution.
From www.chegg.com
Solved A student dilutes 11.5 mL of 6 M NaOH solution with Diluted With Water To Make A Solution The new molarity can easily be calculated by. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. In dilution, we add more solvent to a solution of a higher concentration to. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). We can. Diluted With Water To Make A Solution.
From www.transtutors.com
(Solved) If 10.0 ML Of A 10.0 M Stock Solution Of NaOH Is Diluted Of Diluted With Water To Make A Solution You can easily calculate this value,. When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution. The new molarity can easily be calculated by. You dilute the solution by adding enough water to make the solution volume \(500. For example, if you have a glass. The diluted. Diluted With Water To Make A Solution.
From mmerevise.co.uk
Concentrations and Dilutions MME Diluted With Water To Make A Solution When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution. You can easily calculate this value,. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). The diluted liquid needs to be thoroughly. We can take advantage of. Diluted With Water To Make A Solution.
From slideplayer.com
I. The Nature of Solutions ppt download Diluted With Water To Make A Solution In dilution, we add more solvent to a solution of a higher concentration to. The diluted liquid needs to be thoroughly. You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. The dilution ratio is the ratio of the solute. Diluted With Water To Make A Solution.
From www.showme.com
Concentrated vs. Diluted Solution Science ShowMe Diluted With Water To Make A Solution Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. In dilution, we add more solvent to a solution of a higher concentration to. We can take advantage of dilution to make solutions of. You dilute the solution by adding enough water to make the solution volume \(500. Performing a dilution in chemistry. Diluted With Water To Make A Solution.
From socratic.org
A 10^3 M solution has been diluted to 100 times.Calculate the pH of Diluted With Water To Make A Solution The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent. We can take advantage of dilution to make solutions of. When we add water to a solution, we lower the concentration of the substances. Diluted With Water To Make A Solution.
From www.numerade.com
Calculate the molarity of a solution of FD C Blue Dye No. 1 (MW 792.8 g Diluted With Water To Make A Solution You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. Performing a dilution in chemistry usually means taking a small amount of a. The new molarity can easily be calculated by. We can take advantage of dilution to make solutions. Diluted With Water To Make A Solution.
From ar.inspiredpencil.com
Dilution Orange Juice Diluted With Water To Make A Solution For example, if you have a glass. In dilution, we add more solvent to a solution of a higher concentration to. The new molarity can easily be calculated by. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. You can easily calculate this value,. When we add water to a solution, we. Diluted With Water To Make A Solution.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Diluted With Water To Make A Solution You dilute the solution by adding enough water to make the solution volume \(500. The diluted liquid needs to be thoroughly. Reconstitution is the process of adding a liquid diluent to a dry ingredient to make a solution. In dilution, we add more solvent to a solution of a higher concentration to. When we add water to a solution, we. Diluted With Water To Make A Solution.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture Diluted With Water To Make A Solution When we add water to a solution, we lower the concentration of the substances dissolved in that solution through a process called dilution. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). You dilute the solution by adding enough water to make the solution volume \(500. Performing a dilution in. Diluted With Water To Make A Solution.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Diluted With Water To Make A Solution You can use this calculator to determine how much of it you need if you want to obtain 200 ml of a diluted solution with a concentration of 20 mm. The dilution ratio is the ratio of the solute (the substance to be diluted) to the solvent (e.g., water). You dilute the solution by adding enough water to make the. Diluted With Water To Make A Solution.