Copper(Ii) Nitrate + Sodium Hydroxide Reaction . Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. Approximately 2 ml of solution a (on the left) is added to a. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. Sodium hydroxide precipitates copper(ii) hydroxide: 1 to see which, if any, combination (s) of cation and. You can use ammonia solution instead of sodium hydroxide. When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction.
from www.chegg.com
1 to see which, if any, combination (s) of cation and. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. You can use ammonia solution instead of sodium hydroxide. Approximately 2 ml of solution a (on the left) is added to a. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). Sodium hydroxide precipitates copper(ii) hydroxide: 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution.
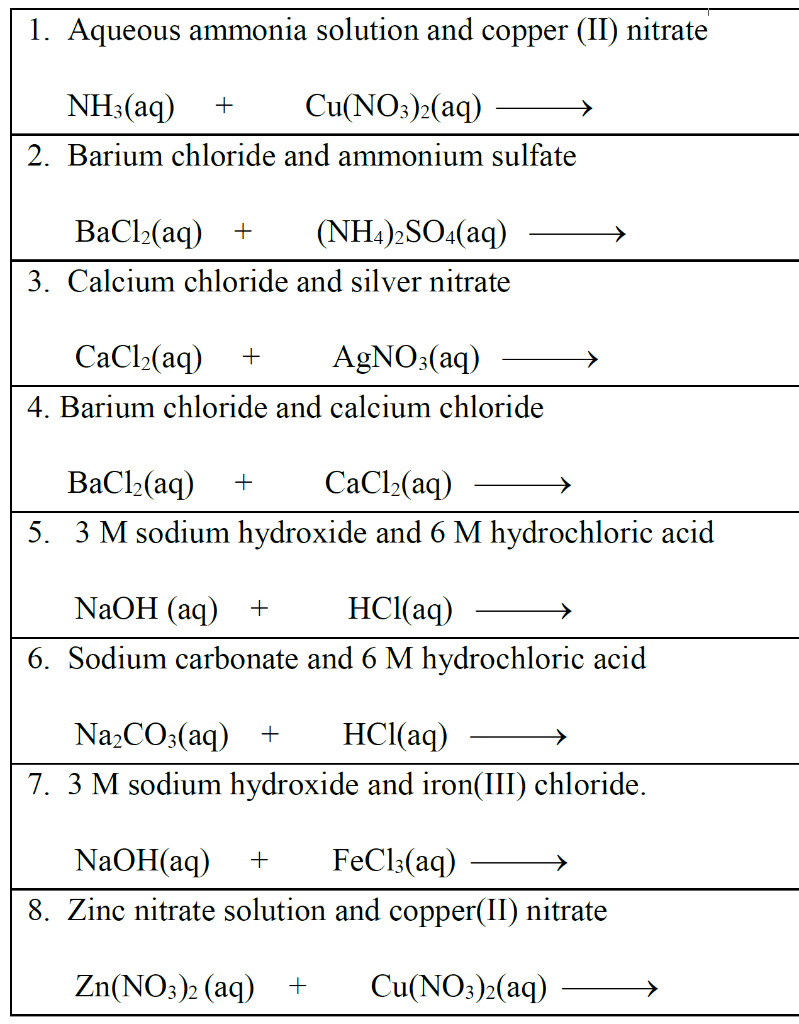
Solved 1. Aqueous ammonia solution and copper (II) nitrate
Copper(Ii) Nitrate + Sodium Hydroxide Reaction You can use ammonia solution instead of sodium hydroxide. When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). Sodium hydroxide precipitates copper(ii) hydroxide: You can use ammonia solution instead of sodium hydroxide. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. 1 to see which, if any, combination (s) of cation and. Approximately 2 ml of solution a (on the left) is added to a. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution.
From www.chegg.com
Solved Lab 5 Chemical Reactions quiz questions Reaction. I Copper(Ii) Nitrate + Sodium Hydroxide Reaction When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. You can use ammonia solution instead of sodium hydroxide. 7 rows solutions containing copper (ii) ions form a. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper(Ii) Nitrate + Sodium Hydroxide Reaction 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). You can use ammonia solution instead of sodium hydroxide. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. By adding. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From nghenhansu.edu.vn
Top 94+ Images Sodium Hydroxide And Copper(ii) Nitrate Sharp Copper(Ii) Nitrate + Sodium Hydroxide Reaction Sodium hydroxide precipitates copper(ii) hydroxide: When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). Approximately 2 ml of solution a (on the left) is added to a. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction.. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper(Ii) Nitrate + Sodium Hydroxide Reaction When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). 1 to see which, if any, combination (s) of cation and. You can use ammonia solution instead of sodium hydroxide. Approximately 2 ml of solution a (on the left) is added to a. By adding basic sodium hydroxide to the. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper(Ii) Nitrate + Sodium Hydroxide Reaction Sodium hydroxide precipitates copper(ii) hydroxide: When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. 1 to see which, if any, combination (s) of cation and.. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.numerade.com
Copper (II) nitrate Sodium phosphate Molecular equation Complete ionic Copper(Ii) Nitrate + Sodium Hydroxide Reaction 1 to see which, if any, combination (s) of cation and. You can use ammonia solution instead of sodium hydroxide. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. Approximately 2 ml of solution a. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper(Ii) Nitrate + Sodium Hydroxide Reaction 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. 1 to see which, if any, combination (s) of cation and. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. When copper (ii) nitrate and sodium hydroxide are mixed, a. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper(Ii) Nitrate + Sodium Hydroxide Reaction When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide precipitates copper(ii) hydroxide: One merely needs to identify all the ions present in the solution and then consider if possible cation/anion. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.chegg.com
Solved 1. Aqueous ammonia solution and copper (II) nitrate Copper(Ii) Nitrate + Sodium Hydroxide Reaction When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). 1 to see which, if any, combination (s) of cation and. You can use ammonia solution instead of sodium hydroxide. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.slideserve.com
PPT End of Semester Review PowerPoint Presentation, free download Copper(Ii) Nitrate + Sodium Hydroxide Reaction To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. You can use ammonia solution instead of sodium hydroxide. Approximately 2 ml of solution. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.youtube.com
How to Balance Cu(NO3)2 + NaOH = Cu(OH)2 + NaNO3 YouTube Copper(Ii) Nitrate + Sodium Hydroxide Reaction One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). You can use ammonia solution instead of sodium hydroxide. Approximately 2 ml of solution a (on. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From fphoto.photoshelter.com
science chemistry precipitation reaction cupric hydroxide Fundamental Copper(Ii) Nitrate + Sodium Hydroxide Reaction 1 to see which, if any, combination (s) of cation and. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. Colorless sodium hydroxide solution is. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED .1873 g of solid metallic copper is fully reacted with nitric Copper(Ii) Nitrate + Sodium Hydroxide Reaction 1 to see which, if any, combination (s) of cation and. Approximately 2 ml of solution a (on the left) is added to a. Sodium hydroxide precipitates copper(ii) hydroxide: When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). To determine whether a precipitation reaction will occur, we identify each. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From socratic.org
What is the net ionic equation for the reaction "NaOH" + "Cu"("NO"_3)_2 Copper(Ii) Nitrate + Sodium Hydroxide Reaction 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. Approximately 2 ml of solution a (on the left) is added to a. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED 1. Write the balanced chemical equation for the reaction of Copper(Ii) Nitrate + Sodium Hydroxide Reaction Approximately 2 ml of solution a (on the left) is added to a. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. 1 to see which, if any, combination (s) of cation and. You can use ammonia solution instead of sodium hydroxide. Sodium hydroxide precipitates copper(ii) hydroxide: By adding basic sodium hydroxide to the solution in your. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.youtube.com
CHEMICAL Reaction .Copper ii Nitrate and Ammonium Hydroxide. YouTube Copper(Ii) Nitrate + Sodium Hydroxide Reaction Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. Approximately 2 ml of solution a (on the left) is added to a. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.slideshare.net
Chemical equations Copper(Ii) Nitrate + Sodium Hydroxide Reaction When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. To determine whether a precipitation reaction will occur, we identify each species in the solution and. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium Copper(Ii) Nitrate + Sodium Hydroxide Reaction To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. One merely needs to identify all the ions present in the solution and then consider if. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.youtube.com
06 Copper nitrate and Sodium hydroxide YouTube Copper(Ii) Nitrate + Sodium Hydroxide Reaction One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. Approximately 2 ml of solution a (on the left) is added to a. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. To determine whether a precipitation reaction. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.slideserve.com
PPT REACTION PREDICTION PreAP CHEMISTRY PowerPoint Presentation Copper(Ii) Nitrate + Sodium Hydroxide Reaction When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). You can use ammonia solution instead of sodium hydroxide. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Approximately 2 ml of solution a (on the left) is added to a. To. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.chegg.com
Solved Aqueous solutions of copper (II) nitrate and sodium Copper(Ii) Nitrate + Sodium Hydroxide Reaction By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. To determine. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED Identify the reactant(s) in the following chemical reaction Cu Copper(Ii) Nitrate + Sodium Hydroxide Reaction 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). To. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Copper(Ii) Nitrate + Sodium Hydroxide Reaction One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED Calculate the number of milliliters of 0.566 M sodium hydroxide Copper(Ii) Nitrate + Sodium Hydroxide Reaction To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. Approximately 2 ml of. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.chegg.com
Solved When copper (II) chloride reacts with sodium nitrate, Copper(Ii) Nitrate + Sodium Hydroxide Reaction 1 to see which, if any, combination (s) of cation and. Approximately 2 ml of solution a (on the left) is added to a. When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.nagwa.com
Question Video Recalling the Color of the Precipitate Forms When Copper(Ii) Nitrate + Sodium Hydroxide Reaction One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From stock.adobe.com
Double displacement reaction sodium hydroxide and copper sulfate Copper(Ii) Nitrate + Sodium Hydroxide Reaction You can use ammonia solution instead of sodium hydroxide. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. 1 to see which, if any, combination (s) of cation and. To determine whether a precipitation reaction will occur, we identify each species in the solution and. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.chegg.com
Solved REACTION 5 COPPER SULFATE (CuSO) AND SODIUM HYDROXIDE Copper(Ii) Nitrate + Sodium Hydroxide Reaction To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. 1 to see which, if any, combination (s) of cation and. You can use ammonia solution instead of sodium hydroxide. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. Approximately 2 ml of solution a. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.youtube.com
How to Write the Net Ionic Equation for NaOH + Cu(NO3)2 = NaNO3 + Cu(OH Copper(Ii) Nitrate + Sodium Hydroxide Reaction Approximately 2 ml of solution a (on the left) is added to a. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. Sodium hydroxide precipitates copper(ii) hydroxide: 1 to see which, if any, combination (s) of cation and. You can use ammonia solution instead of sodium hydroxide. When copper (ii) nitrate and sodium hydroxide are mixed, a. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED Copper (II) nitrate and sodium hydrogen carbonate react Copper(Ii) Nitrate + Sodium Hydroxide Reaction 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. You can use ammonia solution instead of sodium hydroxide. By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. To determine whether a precipitation reaction will occur, we identify each species. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.numerade.com
SOLVED a. What is the net ionic equation for silver nitrate + calcium Copper(Ii) Nitrate + Sodium Hydroxide Reaction 1 to see which, if any, combination (s) of cation and. One merely needs to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble. 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. Sodium hydroxide precipitates copper(ii) hydroxide: You can use. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.chegg.com
Solved 1. Write balanced chemical equations for each Copper(Ii) Nitrate + Sodium Hydroxide Reaction 7 rows solutions containing copper (ii) ions form a blue precipitate when mixed with sodium hydroxide solution. When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). 1 to see which, if any, combination (s) of cation and. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution.. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper(Ii) Nitrate + Sodium Hydroxide Reaction Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. 1 to see which, if any, combination (s) of cation and. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. Approximately 2 ml of solution a (on the left) is added to a. Sodium hydroxide. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.youtube.com
2. Copper Nitrate to Copper Hydroxide (Cu Again Lab) YouTube Copper(Ii) Nitrate + Sodium Hydroxide Reaction By adding basic sodium hydroxide to the solution in your beaker, it will first destroy the leftover acid from the previous reaction. Colorless sodium hydroxide solution is added to blue copper (ii) nitrate solution. Sodium hydroxide precipitates copper(ii) hydroxide: When copper (ii) nitrate and sodium hydroxide are mixed, a chemical reaction occurs, resulting in the formation of copper (ii). Approximately. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper(Ii) Nitrate + Sodium Hydroxide Reaction 1 to see which, if any, combination (s) of cation and. Approximately 2 ml of solution a (on the left) is added to a. To determine whether a precipitation reaction will occur, we identify each species in the solution and then refer to table 4.5.1 4.5. One merely needs to identify all the ions present in the solution and then. Copper(Ii) Nitrate + Sodium Hydroxide Reaction.