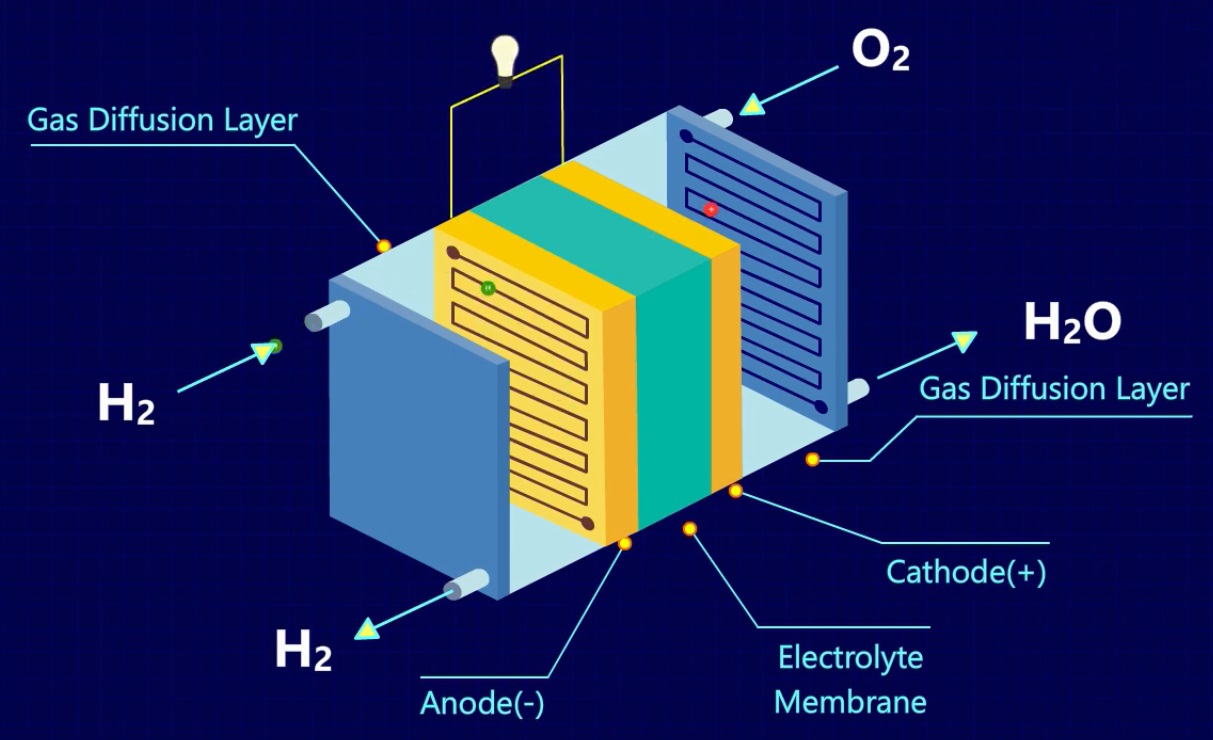
Chemistry Behind Fuel Cells . A fuel cell has three main parts: this post will look at the chemistry behind fuel cells and the different fuel cell variations that have been developed to support a. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. so how does it all work? in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In this section, we describe the. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells work by converting.
from www.electroniclinic.com
in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. this post will look at the chemistry behind fuel cells and the different fuel cell variations that have been developed to support a. A fuel cell has three main parts: In this section, we describe the. so how does it all work? a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. Fuel cells work by converting. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available.
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working
Chemistry Behind Fuel Cells so how does it all work? A fuel cell has three main parts: so how does it all work? fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells work by converting. this post will look at the chemistry behind fuel cells and the different fuel cell variations that have been developed to support a. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. In this section, we describe the.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Chemistry Behind Fuel Cells A fuel cell has three main parts: so how does it all work? fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. a fuel. Chemistry Behind Fuel Cells.
From iupac.org
Fuel Cells IUPAC International Union of Pure and Applied Chemistry Chemistry Behind Fuel Cells a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. In this section, we describe the. Fuel cells work by converting. a fuel cell. Chemistry Behind Fuel Cells.
From large.stanford.edu
Hydrogen Fuel Cells Chemistry Behind Fuel Cells Fuel cells work by converting. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel cell has three main parts: in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. fuels cells. Chemistry Behind Fuel Cells.
From revisechemistry.uk
Chemical Cells and Fuel Cells AQA C5 revisechemistry.uk Chemistry Behind Fuel Cells In this section, we describe the. A fuel cell has three main parts: Fuel cells work by converting. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to. Chemistry Behind Fuel Cells.
From www.change-florida.com
Hydrogen Fuel Cells Chemistry Behind Fuel Cells fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. this post will look at the chemistry behind fuel cells and the different fuel cell variations that have been developed to support a. A fuel cell has three main parts: Fuel cells work by converting. so how does it. Chemistry Behind Fuel Cells.
From www.youtube.com
Introduction to Types of Fuel Cells YouTube Chemistry Behind Fuel Cells a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. this post will look at the chemistry behind fuel cells and the different. Chemistry Behind Fuel Cells.
From chemistrycommunity.nature.com
Triple phase boundary in solid oxide fuel cells Reaction dynamics Chemistry Behind Fuel Cells a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. so how does it all work? this post will look at the chemistry behind. Chemistry Behind Fuel Cells.
From chem.libretexts.org
Case Study Fuel Cells Chemistry LibreTexts Chemistry Behind Fuel Cells a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. In this section, we describe the. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells work by converting. fuels cells generate electricity. Chemistry Behind Fuel Cells.
From www.researchgate.net
Basic structure of PEM fuel cell Download Scientific Diagram Chemistry Behind Fuel Cells so how does it all work? fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. this post will look at the chemistry behind fuel cells and the. Chemistry Behind Fuel Cells.
From courses.lumenlearning.com
Phases and Classification of Matter Introductory Chemistry Lecture Chemistry Behind Fuel Cells in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. In this section, we describe the. Fuel cells work by converting. so how does it all work? a fuel cell like this will continue to operate and produce electrical energy as long as a. Chemistry Behind Fuel Cells.
From www.youtube.com
Hydrogen & Fuel Cells Reactions Chemistry FuseSchool YouTube Chemistry Behind Fuel Cells a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. a fuel cell like this will continue to operate and produce electrical energy as long as. Chemistry Behind Fuel Cells.
From studymind.co.uk
Fuel Cells (GCSE Chemistry) Study Mind Chemistry Behind Fuel Cells In this section, we describe the. so how does it all work? in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. Fuel cells work by converting. A fuel cell has three main parts: a fuel cell consists of two electrodes—a negative electrode (or. Chemistry Behind Fuel Cells.
From blogs.sw.siemens.com
Hydrogen fuel cell modeling (almost) all you need to know Simcenter Chemistry Behind Fuel Cells in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. this post will look at the chemistry behind fuel cells and the different fuel cell variations that have been developed to support a. In this section, we describe the. a fuel cell consists of. Chemistry Behind Fuel Cells.
From greenfuture.io
What Are Hydrogen Fuel Cell Cars? Chemistry Behind Fuel Cells a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode. Chemistry Behind Fuel Cells.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Chemistry Behind Fuel Cells so how does it all work? a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cells work by converting. A fuel cell has three main parts: . Chemistry Behind Fuel Cells.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Chemistry Behind Fuel Cells A fuel cell has three main parts: In this section, we describe the. Fuel cells work by converting. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently. Chemistry Behind Fuel Cells.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Chemistry Behind Fuel Cells in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuels cells generate electricity from an electrochemical reaction in which. Chemistry Behind Fuel Cells.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Chemistry Behind Fuel Cells fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. so how does it all work? A fuel cell has three main parts: a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Fuel cells work by converting. in contrast,. Chemistry Behind Fuel Cells.
From www.e-conversion.de
Fuel cell principle econversion Chemistry Behind Fuel Cells fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. In this section, we describe the. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. this post will look at the chemistry behind fuel cells and the different fuel cell. Chemistry Behind Fuel Cells.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Chemistry Behind Fuel Cells a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. so how does it all work? In this section, we describe the. this post will. Chemistry Behind Fuel Cells.
From www.ki.si
GrapheneDerived Carbon Support Boosts Proton Exchange Membrane Fuel Chemistry Behind Fuel Cells a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell has three main parts: fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. a fuel cell uses the chemical energy of. Chemistry Behind Fuel Cells.
From www.chfca.ca
About Fuel Cells CHFCA Chemistry Behind Fuel Cells in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell has three main parts: so how does it all work? Fuel cells work. Chemistry Behind Fuel Cells.
From www.tes.com
Electrolysis and Fuel Cells GCSE Chemistry Teaching Resources Chemistry Behind Fuel Cells a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. Fuel cells work by converting. this post will look at the chemistry behind fuel cells. Chemistry Behind Fuel Cells.
From pressbooks.openedmb.ca
Batteries and Fuel Cells Chemistry and the Environment Chemistry Behind Fuel Cells so how does it all work? Fuel cells work by converting. fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell like this will continue to operate. Chemistry Behind Fuel Cells.
From stock.adobe.com
Fuel Cell Solid Oxide Diagram Stock Vector Adobe Stock Chemistry Behind Fuel Cells a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. so how does it all work? a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. Fuel cells work by converting. a. Chemistry Behind Fuel Cells.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne Chemistry Behind Fuel Cells A fuel cell has three main parts: a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. so how does. Chemistry Behind Fuel Cells.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink Chemistry Behind Fuel Cells a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more. Chemistry Behind Fuel Cells.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Chemistry Behind Fuel Cells so how does it all work? fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. in contrast, a fuel cell is a galvanic. Chemistry Behind Fuel Cells.
From chem.libretexts.org
20.7 Batteries and Fuel Cells Chemistry LibreTexts Chemistry Behind Fuel Cells so how does it all work? A fuel cell has three main parts: a fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. this post will look at. Chemistry Behind Fuel Cells.
From www.youtube.com
GCSE Chemistry Fuel Cells YouTube Chemistry Behind Fuel Cells a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. so how does it all work? Fuel cells work by converting. In this section, we describe the. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen. Chemistry Behind Fuel Cells.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip Chemistry Behind Fuel Cells In this section, we describe the. Fuel cells work by converting. A fuel cell has three main parts: in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. this post will look at the chemistry behind fuel cells and the different fuel cell variations that. Chemistry Behind Fuel Cells.
From www.youtube.com
AQA GCSE Chemistry P1 Hydrogen Fuel Cells YouTube Chemistry Behind Fuel Cells a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel cell has three main parts: a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. in contrast, a fuel cell is a galvanic. Chemistry Behind Fuel Cells.
From www.youtube.com
GCSE Chemistry Fuel Cells 45 YouTube Chemistry Behind Fuel Cells In this section, we describe the. a fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell has three main parts: so how does it all work? a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly. Chemistry Behind Fuel Cells.
From www.dataphysics-instruments.com
How measuring contact angles at high temperatures can help develop fuel Chemistry Behind Fuel Cells this post will look at the chemistry behind fuel cells and the different fuel cell variations that have been developed to support a. in contrast, a fuel cell is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity. a fuel cell consists of two electrodes—a negative electrode (or anode). Chemistry Behind Fuel Cells.
From www.narayannepal.com.np
Electrochemistry Class 12 Chemistry Notes NEB Best NEB Notes Chemistry Behind Fuel Cells A fuel cell has three main parts: this post will look at the chemistry behind fuel cells and the different fuel cell variations that have been developed to support a. so how does it all work? a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel. Chemistry Behind Fuel Cells.