How Does A Catalyst Work In A Chemical Reaction . A catalyst does not alter the position of equilibrium in a reversible reaction. Learn how catalysts work, what they look like and why they are. The catalyst is generally specific in its action. Learn how catalysts work, types of catalysts, and examples of. However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. Without a catalyst, this reaction is very slow. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Learn about the three major classes of. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn about the different types of catalysts, how they work, and. A catalyst is a material that speeds up chemical reactions by lowering the activation energy.
from newenergyandfuel.com
However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. The catalyst is generally specific in its action. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. A catalyst does not alter the position of equilibrium in a reversible reaction. Without a catalyst, this reaction is very slow. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Learn how catalysts work, what they look like and why they are. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Learn about the different types of catalysts, how they work, and.
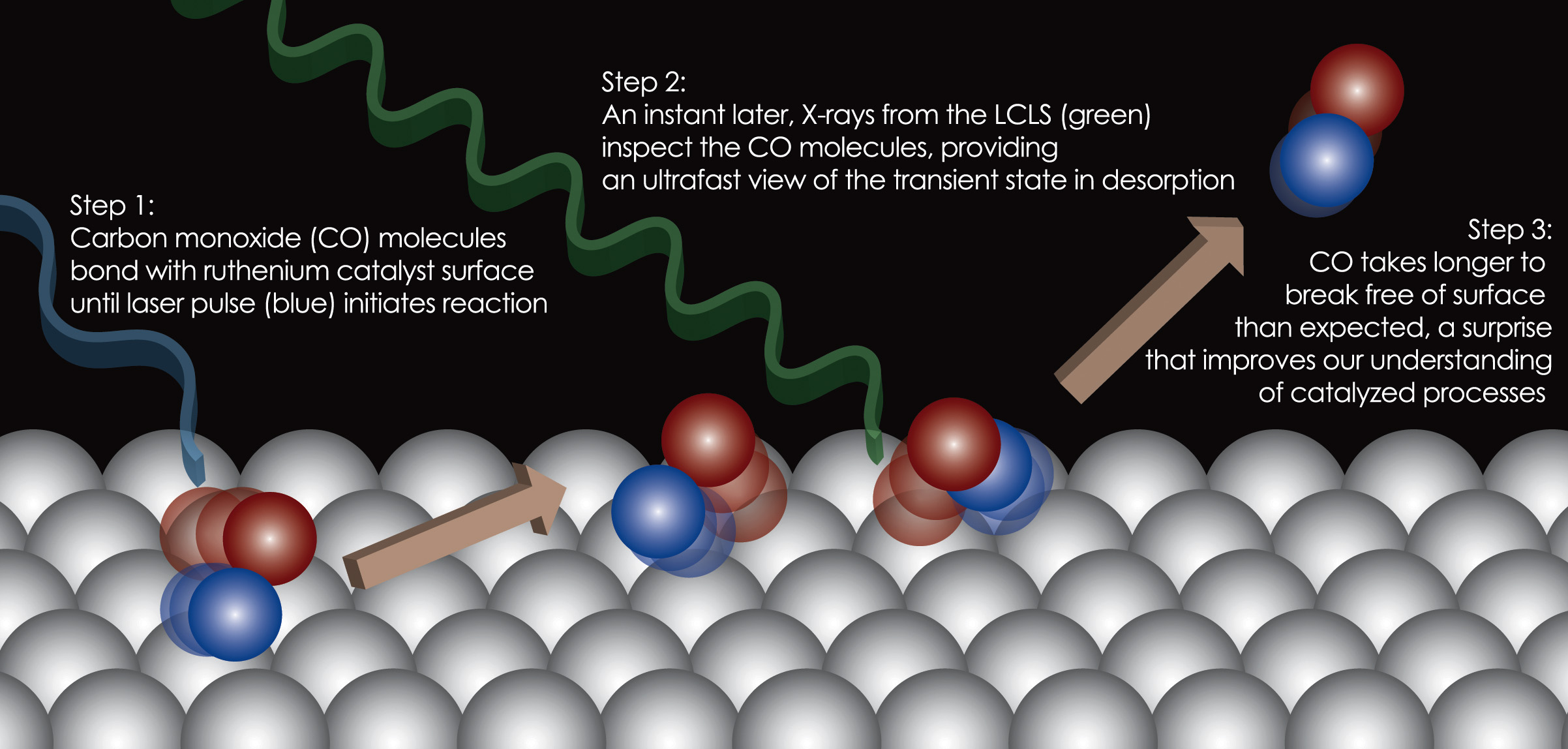
Seeing a Catalytic Chemical Reaction in Real Time
How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn how catalysts work, what they look like and why they are. A catalyst does not alter the position of equilibrium in a reversible reaction. Without a catalyst, this reaction is very slow. The catalyst is generally specific in its action. Learn about the different types of catalysts, how they work, and. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn how catalysts work, types of catalysts, and examples of. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Learn about the three major classes of.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn how catalysts work, what they look like and why they are. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about the different types of catalysts, how they work, and. The. How Does A Catalyst Work In A Chemical Reaction.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation How Does A Catalyst Work In A Chemical Reaction Learn about the three major classes of. Without a catalyst, this reaction is very slow. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn how catalysts work, what they look like and why they are. Learn about the different types of catalysts, how they work, and. A catalyst is a. How Does A Catalyst Work In A Chemical Reaction.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste How Does A Catalyst Work In A Chemical Reaction A catalyst does not alter the position of equilibrium in a reversible reaction. Learn about the different types of catalysts, how they work, and. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst is a substance that increases the rate of a. How Does A Catalyst Work In A Chemical Reaction.
From hxefjeszm.blob.core.windows.net
What Role Do Catalysts Play In Chemical Reactions at Andre er blog How Does A Catalyst Work In A Chemical Reaction The catalyst is generally specific in its action. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Without a catalyst, this reaction is very slow. However,. How Does A Catalyst Work In A Chemical Reaction.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Learn how catalysts work, what they look like and why they are. Learn how catalysts work, types of catalysts, and. How Does A Catalyst Work In A Chemical Reaction.
From www.slideserve.com
PPT Fast and Slow Chemistry PowerPoint Presentation, free download How Does A Catalyst Work In A Chemical Reaction Learn about the different types of catalysts, how they work, and. However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Without a catalyst, this reaction is very slow. Catalysis is the process of using substances. How Does A Catalyst Work In A Chemical Reaction.
From www.youtube.com
Effect of Catalyst on Rate of Reaction Chemical Chemistry How Does A Catalyst Work In A Chemical Reaction The catalyst is generally specific in its action. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Learn how catalysts work, what they look like and why they are. Without a catalyst, this reaction is very slow. Learn about the three major classes of.. How Does A Catalyst Work In A Chemical Reaction.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube How Does A Catalyst Work In A Chemical Reaction A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Learn about the three major classes of. Learn how catalysts work, what they look like and why they are. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. However,. How Does A Catalyst Work In A Chemical Reaction.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. The catalyst is generally specific in its action. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed. How Does A Catalyst Work In A Chemical Reaction.
From www.pinterest.com
Catalyst definition A substance that speeds up a chemical reaction by How Does A Catalyst Work In A Chemical Reaction Learn about the three major classes of. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Learn about the different types of catalysts, how they work, and. Without a catalyst, this reaction is very slow. A catalyst is a substance that increases the rate of a chemical. How Does A Catalyst Work In A Chemical Reaction.
From giohrwthv.blob.core.windows.net
Catalyst Do In Chemical Reactions at Rosa Baugher blog How Does A Catalyst Work In A Chemical Reaction The catalyst is generally specific in its action. Learn about the three major classes of. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a substance. How Does A Catalyst Work In A Chemical Reaction.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. The catalyst is generally specific in its action. A catalyst does not alter the position of equilibrium in a reversible reaction. Learn about the different types of catalysts, how they work, and. A catalyst is. How Does A Catalyst Work In A Chemical Reaction.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram How Does A Catalyst Work In A Chemical Reaction Learn how catalysts work, types of catalysts, and examples of. However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. A catalyst does not alter the position of equilibrium in a reversible reaction. Learn how catalysts work, what they look like and why they are. Without a catalyst, this reaction is very slow. Learn. How Does A Catalyst Work In A Chemical Reaction.
From www.youtube.com
Heterogeneous catalyst & catalysis 12th Std Chemistry Science How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn how catalysts work, what they look like and why they are. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a substance that increases the. How Does A Catalyst Work In A Chemical Reaction.
From www.youtube.com
Identifying catalysts in a reaction YouTube How Does A Catalyst Work In A Chemical Reaction A catalyst is a material that speeds up chemical reactions by lowering the activation energy. However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. Learn how catalysts work, what they look like and why they are. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without. How Does A Catalyst Work In A Chemical Reaction.
From study.com
Effect of Catalysts on Rates of Reaction Lesson How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn how catalysts work, what they look like and why they are. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Learn how catalysts work, types of catalysts, and examples of. Without a catalyst, this reaction. How Does A Catalyst Work In A Chemical Reaction.
From diagramlibdrefnwyrzyf.z13.web.core.windows.net
Catalyst Diagram Chemistry How Does A Catalyst Work In A Chemical Reaction However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. Learn how catalysts work, types of catalysts, and examples of. The catalyst is generally specific in its action. Without a catalyst, this reaction is very slow. A catalyst does not alter the position of equilibrium in a reversible reaction. A catalyst is a substance. How Does A Catalyst Work In A Chemical Reaction.
From dxojlxtmb.blob.core.windows.net
How Do Enzymes Act As Biological Catalysts (Choose 3) at Robert How Does A Catalyst Work In A Chemical Reaction Learn how catalysts work, what they look like and why they are. However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. A catalyst is a substance that increases the rate of a chemical reaction by. How Does A Catalyst Work In A Chemical Reaction.
From socratic.org
What will occur if a catalyst is added to a reaction mixture? Socratic How Does A Catalyst Work In A Chemical Reaction A catalyst is a material that speeds up chemical reactions by lowering the activation energy. A catalyst does not alter the position of equilibrium in a reversible reaction. Learn about the different types of catalysts, how they work, and. However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. A catalyst is a substance. How Does A Catalyst Work In A Chemical Reaction.
From www.linstitute.net
IB DP Chemistry HL复习笔记6.1.8 Energy Profiles & Catalysis翰林国际教育 How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn about the three major classes of. The catalyst is generally specific in its action. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst. How Does A Catalyst Work In A Chemical Reaction.
From www.slideshare.net
Biology 2.4 How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn about the three major classes of. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. Without a catalyst, this reaction is very slow. However, when the reaction is. How Does A Catalyst Work In A Chemical Reaction.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. The catalyst is generally specific in its action. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. Learn how catalysts. How Does A Catalyst Work In A Chemical Reaction.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube How Does A Catalyst Work In A Chemical Reaction However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Learn about the different types of catalysts, how they work, and. Learn how catalysts work, types of catalysts, and examples of. Without a catalyst, this reaction is very slow.. How Does A Catalyst Work In A Chemical Reaction.
From giofwvfzm.blob.core.windows.net
How Does A Catalyst Work To Speed Up A Reaction at William Chambless blog How Does A Catalyst Work In A Chemical Reaction Learn about the different types of catalysts, how they work, and. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. A catalyst is a substance that. How Does A Catalyst Work In A Chemical Reaction.
From www.youtube.com
How does a CATALYST work ? YouTube How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn how catalysts work, what they look like and why they are. Learn how catalysts work, types of catalysts, and examples of. A catalyst does not alter the position of equilibrium in a reversible reaction. A catalyst is a material that speeds. How Does A Catalyst Work In A Chemical Reaction.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii How Does A Catalyst Work In A Chemical Reaction Learn about the different types of catalysts, how they work, and. Learn about the three major classes of. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn how catalysts work, what they. How Does A Catalyst Work In A Chemical Reaction.
From newenergyandfuel.com
Seeing a Catalytic Chemical Reaction in Real Time How Does A Catalyst Work In A Chemical Reaction Learn how catalysts work, what they look like and why they are. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. However, when the reaction is catalyzed by a. How Does A Catalyst Work In A Chemical Reaction.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst does not alter the position of equilibrium in a reversible reaction. A catalyst is a material that speeds. How Does A Catalyst Work In A Chemical Reaction.
From giohrwthv.blob.core.windows.net
Catalyst Do In Chemical Reactions at Rosa Baugher blog How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. The catalyst is generally specific in its action. Learn how catalysts work, what they look like and why they are. A catalyst is a. How Does A Catalyst Work In A Chemical Reaction.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Without a catalyst, this reaction is very slow. Learn how catalysts work, what they look like and why they are. A catalyst is a material that speeds up chemical reactions by lowering the activation energy.. How Does A Catalyst Work In A Chemical Reaction.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 How Does A Catalyst Work In A Chemical Reaction However, when the reaction is catalyzed by a metal such as nickel, palladium or platinum, the. Learn how catalysts work, what they look like and why they are. Learn about the three major classes of. Learn how catalysts work, types of catalysts, and examples of. Without a catalyst, this reaction is very slow. Learn about the different types of catalysts,. How Does A Catalyst Work In A Chemical Reaction.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 How Does A Catalyst Work In A Chemical Reaction Learn how catalysts work, what they look like and why they are. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Without a catalyst, this reaction is very slow. Learn about the three major classes of. Catalysis is the process of using substances that. How Does A Catalyst Work In A Chemical Reaction.
From ch302.cm.utexas.edu
chem1 How Does A Catalyst Work In A Chemical Reaction Learn about the different types of catalysts, how they work, and. Learn how catalysts work, types of catalysts, and examples of. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Catalysis is the process of using substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst. How Does A Catalyst Work In A Chemical Reaction.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram How Does A Catalyst Work In A Chemical Reaction A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. A catalyst does not alter the position of equilibrium in a reversible reaction. The catalyst is generally specific in its action. Without a catalyst, this reaction is very slow. A catalyst is a substance that increases the rate of a chemical reaction. How Does A Catalyst Work In A Chemical Reaction.
From www.mdpi.com
Catalysts Free FullText Intensification of Catalytic Processes How Does A Catalyst Work In A Chemical Reaction A catalyst does not alter the position of equilibrium in a reversible reaction. A catalyst is a substance that affects the rate of a chemical reaction by lowering its activation energy. Learn how catalysts work, what they look like and why they are. Learn how catalysts work, types of catalysts, and examples of. Learn about the different types of catalysts,. How Does A Catalyst Work In A Chemical Reaction.