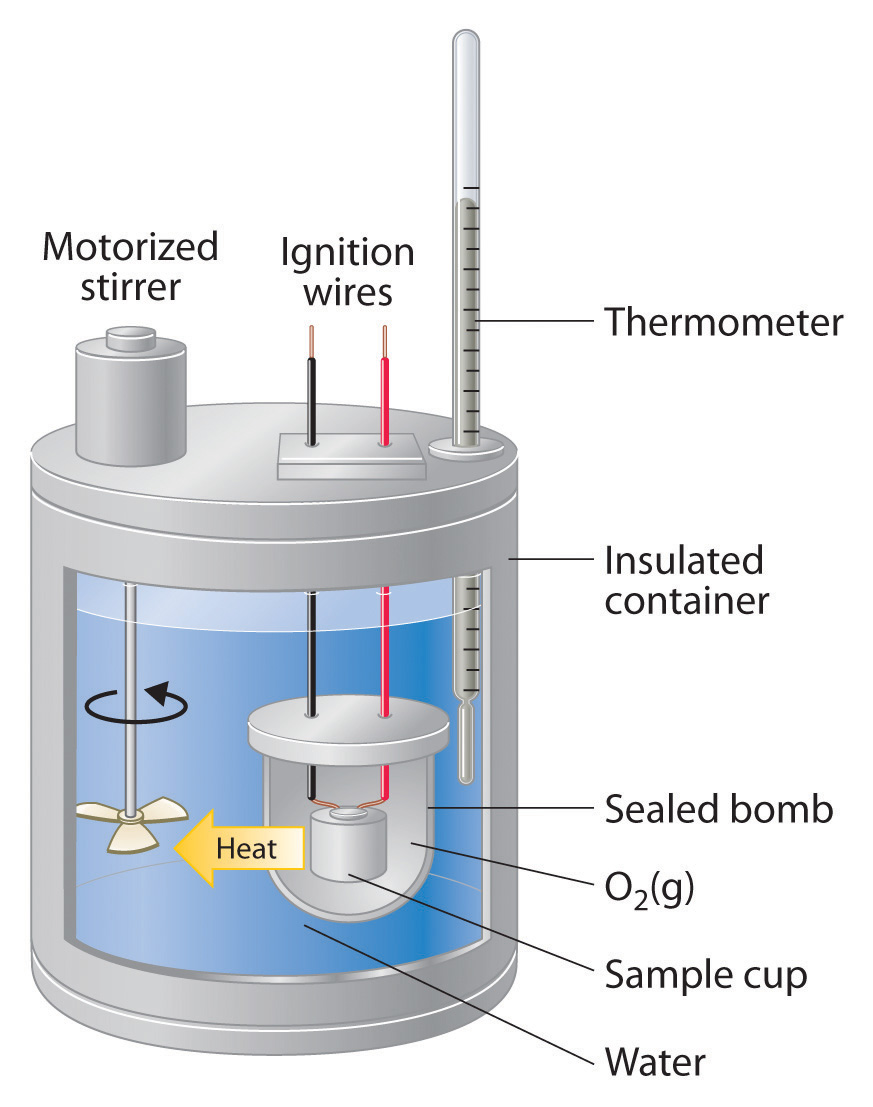
Calorimetry Law Definition . The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. By knowing the change in. To do so, the heat is exchanged with a calibrated object (calorimeter). Compare heat flow from hot to cold objects in an ideal. Chemists use calorimetry to determine the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends.
from sites.google.com
Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. By knowing the change in. For example, when an exothermic. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Compare heat flow from hot to cold objects in an ideal. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must.
Calorimetry Preliminary HSC Chemistry
Calorimetry Law Definition The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. To do so, the heat is exchanged with a calibrated object (calorimeter). Compare heat flow from hot to cold objects in an ideal. By knowing the change in. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Chemists use calorimetry to determine the. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Law Definition For example, when an exothermic. Calorimetry is used to measure amounts of heat transferred to or from a substance. By knowing the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. To do so, the heat is. Calorimetry Law Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Law Definition Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must.. Calorimetry Law Definition.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Law Definition The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. For example, when an exothermic. To do so, the heat is exchanged with a calibrated object (calorimeter). Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or. Calorimetry Law Definition.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Law Definition Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Apply the first law of thermodynamics to calorimetry. By knowing the change in. To do so, the heat is exchanged with a calibrated object (calorimeter). The basic principle of calorimetry is the law of conservation of energy which states that the sum of. Calorimetry Law Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Law Definition Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or. Calorimetry Law Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Law Definition The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. To do so, the heat is exchanged with a calibrated object (calorimeter). Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of. Calorimetry Law Definition.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Law Definition To do so, the heat is exchanged with a calibrated object (calorimeter). Apply the first law of thermodynamics to calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic. By. Calorimetry Law Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Law Definition Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Compare heat flow from hot to cold objects in an ideal. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in. Calorimetry Law Definition.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Law Definition Calorimetry is used to measure amounts of heat transferred to or from a substance. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Apply the first. Calorimetry Law Definition.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Law Definition Chemists use calorimetry to determine the. Apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a. Calorimetry Law Definition.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Law Definition Compare heat flow from hot to cold objects in an ideal. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. By knowing the change in. Chemists use calorimetry to determine. Calorimetry Law Definition.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Law Definition Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. Calorimetry measures enthalpy changes during. Calorimetry Law Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Law Definition Chemists use calorimetry to determine the. Compare heat flow from hot to cold objects in an ideal. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. By knowing the change in. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of. Calorimetry Law Definition.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Law Definition Calorimetry is used to measure amounts of heat transferred to or from a substance. By knowing the change in. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. For example,. Calorimetry Law Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Law Definition The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used to measure the amount of heat. Calorimetry Law Definition.
From www.aquaportail.com
Calorimétrie définition et explications Calorimetry Law Definition For example, when an exothermic. By knowing the change in. Apply the first law of thermodynamics to calorimetry. Chemists use calorimetry to determine the. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all. Calorimetry Law Definition.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimetry Law Definition Compare heat flow from hot to cold objects in an ideal. To do so, the heat is exchanged with a calibrated object (calorimeter). For example, when an exothermic. Calorimetry is used to measure amounts of heat transferred to or from a substance. By knowing the change in. Apply the first law of thermodynamics to calorimetry. The basic principle of calorimetry. Calorimetry Law Definition.
From www.youtube.com
Introduction à la calorimétrie YouTube Calorimetry Law Definition Chemists use calorimetry to determine the. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system. Calorimetry Law Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Law Definition Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. The basic principle of calorimetry is the law of conservation of energy which states. Calorimetry Law Definition.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Law Definition Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Chemists use calorimetry to determine the. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. The basic principle of calorimetry is the law of conservation of energy which states that the sum. Calorimetry Law Definition.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample Calorimetry Law Definition To do so, the heat is exchanged with a calibrated object (calorimeter). Compare heat flow from hot to cold objects in an ideal. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is the process of measuring the amount. Calorimetry Law Definition.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Law Definition Chemists use calorimetry to determine the. Apply the first law of thermodynamics to calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. By knowing the change in. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Compare heat. Calorimetry Law Definition.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Law Definition Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. To do. Calorimetry Law Definition.
From ar.inspiredpencil.com
Calorimetry Equation Calorimetry Law Definition Compare heat flow from hot to cold objects in an ideal. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. By. Calorimetry Law Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Law Definition Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Compare heat flow from hot to cold objects in an ideal. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when. Calorimetry Law Definition.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry Law Definition By knowing the change in. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. A calorimeter is a. Calorimetry Law Definition.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Law Definition By knowing the change in. For example, when an exothermic. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal. Chemists use calorimetry to determine the. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calorimetry is a field of thermochemistry that measures the amount. Calorimetry Law Definition.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimetry Law Definition For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Apply the first law of thermodynamics to calorimetry. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. Calorimetry is the process. Calorimetry Law Definition.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 Calorimetry Law Definition The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. Chemists use calorimetry to determine the. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry. Calorimetry Law Definition.
From studylib.net
Calorimetry Calorimetry Law Definition The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. A calorimeter. Calorimetry Law Definition.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Law Definition To do so, the heat is exchanged with a calibrated object (calorimeter). By knowing the change in. Calorimetry is used to measure amounts of heat transferred to or from a substance. For example, when an exothermic. Chemists use calorimetry to determine the. The basic principle of calorimetry is the law of conservation of energy which states that the sum of. Calorimetry Law Definition.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Law Definition Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Calorimetry Law Definition.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Law Definition For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a system must. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Calorimetry Law Definition.
From www.slideserve.com
PPT 18 Heat and the First Law of Thermodynamics PowerPoint Calorimetry Law Definition For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure amounts of heat transferred to or from a. Calorimetry Law Definition.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Law Definition Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic. Calorimetry is used to measure amounts of heat transferred to or from a substance. The basic principle of calorimetry is the law of conservation of energy which states that the sum of all energies in a. Calorimetry Law Definition.