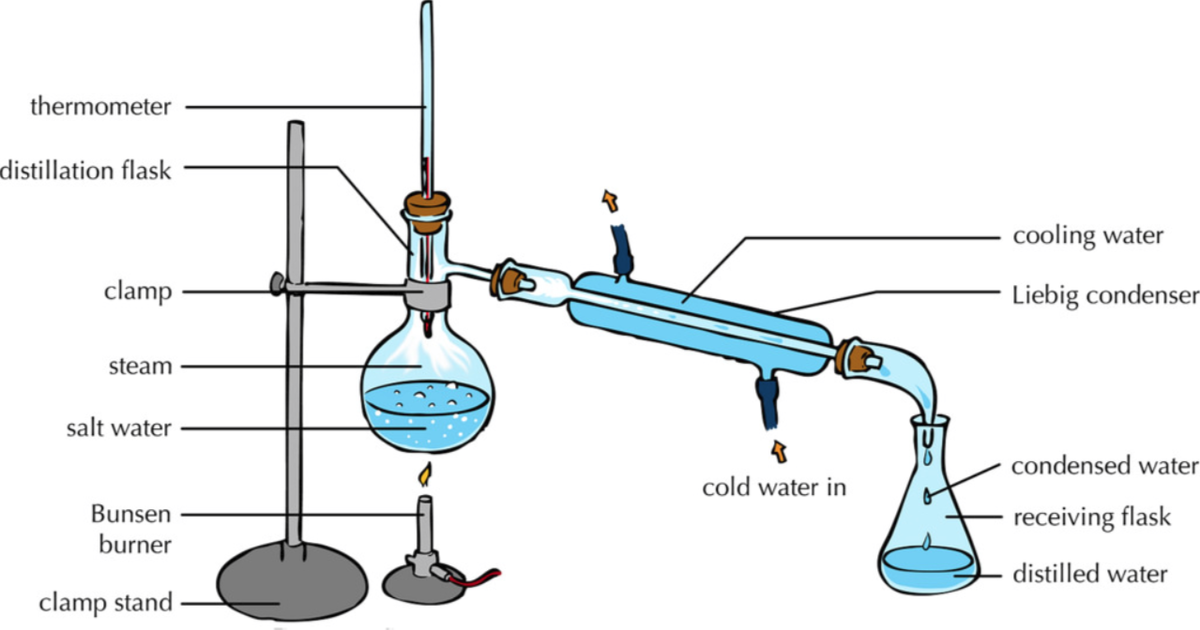
Distillation Lab Experiment . In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. In this experiment, we will separate 2 distillates using their differences in boiling points. Collect distillate at a rate of 1 drop per second. Obtain a mass spectrum and a gas chromatogram of your. Perform atmospheric pressure distillation—see distillation guide. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Record the temperature where liquid is. The boiling points of individual. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Apply the heat source to the distilling flask. Turn on the condenser water. Investigate the separation of water from a coloured solution using this simple distillation video, including.
from keystagewiki.com
Record the temperature where liquid is. Turn on the condenser water. Obtain a mass spectrum and a gas chromatogram of your. The boiling points of individual. Perform atmospheric pressure distillation—see distillation guide. Collect distillate at a rate of 1 drop per second. In this experiment, we will separate 2 distillates using their differences in boiling points. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Apply the heat source to the distilling flask. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions.
Distillation Key Stage Wiki
Distillation Lab Experiment The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Turn on the condenser water. The boiling points of individual. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Obtain a mass spectrum and a gas chromatogram of your. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Apply the heat source to the distilling flask. Record the temperature where liquid is. Investigate the separation of water from a coloured solution using this simple distillation video, including. Perform atmospheric pressure distillation—see distillation guide. In this experiment, we will separate 2 distillates using their differences in boiling points. Collect distillate at a rate of 1 drop per second. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids.
From keystagewiki.com
Distillation Key Stage Wiki Distillation Lab Experiment Obtain a mass spectrum and a gas chromatogram of your. Investigate the separation of water from a coloured solution using this simple distillation video, including. In this experiment, we will separate 2 distillates using their differences in boiling points. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids.. Distillation Lab Experiment.
From www.docsity.com
Simple and fractional distillation lab report Docsity Distillation Lab Experiment Apply the heat source to the distilling flask. Investigate the separation of water from a coloured solution using this simple distillation video, including. Collect distillate at a rate of 1 drop per second. The boiling points of individual. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Perform. Distillation Lab Experiment.
From www.turbosquid.com
3d Distillation Lab Experiment Model Distillation Lab Experiment Perform atmospheric pressure distillation—see distillation guide. Apply the heat source to the distilling flask. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. The boiling points of individual. Collect distillate at a rate of 1 drop per second. Turn on the condenser water. In this experiment, we will separate 2 distillates using. Distillation Lab Experiment.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Distillation Lab Experiment The boiling points of individual. Apply the heat source to the distilling flask. Investigate the separation of water from a coloured solution using this simple distillation video, including. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Turn on the condenser water. In this experiment, we will separate. Distillation Lab Experiment.
From chem.libretexts.org
5.5D StepbyStep Procedures for Steam Distillation Chemistry LibreTexts Distillation Lab Experiment Investigate the separation of water from a coloured solution using this simple distillation video, including. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Collect distillate at a rate of. Distillation Lab Experiment.
From studylib.net
Steam Distillation Experiment Distillation Lab Experiment The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. Record the temperature where liquid is. Distillation is one of the oldest and still most common methods for both the purification and the identification of. Distillation Lab Experiment.
From www.turbosquid.com
3d Distillation Lab Experiment Model Distillation Lab Experiment Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Investigate the separation of water from a coloured solution using this simple distillation video, including. Turn on the condenser water. Obtain a mass spectrum and a gas chromatogram of your. Record the temperature where liquid is. Perform atmospheric pressure. Distillation Lab Experiment.
From www.youtube.com
Introduction to Fractional distillation Distillation procedure Home Distillation Lab Experiment Investigate the separation of water from a coloured solution using this simple distillation video, including. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Record the temperature where liquid is. Apply the heat source to the distilling flask. Obtain a mass spectrum and a gas chromatogram of your.. Distillation Lab Experiment.
From chem.libretexts.org
5.3A Theory of Fractional Distillation Chemistry LibreTexts Distillation Lab Experiment In this experiment, we will separate 2 distillates using their differences in boiling points. Apply the heat source to the distilling flask. Turn on the condenser water. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Investigate the separation of water from a coloured solution using this. Distillation Lab Experiment.
From www.thoughtco.com
What Is Distillation? Principles and Uses Distillation Lab Experiment Turn on the condenser water. Investigate the separation of water from a coloured solution using this simple distillation video, including. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Collect distillate at a rate of 1 drop per second. Record the temperature where liquid is. Apply the. Distillation Lab Experiment.
From www.turbosquid.com
3d Distillation Lab Experiment Model Distillation Lab Experiment Apply the heat source to the distilling flask. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Investigate the separation of water from a coloured solution using this simple distillation video, including. Collect distillate at a rate of 1 drop per second. In this experiment, you will be conducting both a simple. Distillation Lab Experiment.
From ceahjckh.blob.core.windows.net
How To Make Distilled Water In Chemistry Lab at Byron Taylor blog Distillation Lab Experiment Turn on the condenser water. Collect distillate at a rate of 1 drop per second. Perform atmospheric pressure distillation—see distillation guide. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. In this experiment, we will separate 2 distillates using their differences in boiling points. Obtain a mass spectrum and a gas chromatogram. Distillation Lab Experiment.
From tnlab.com
5 Liter Short Path Distillation System TN Lab Supply, LLC Distillation Lab Experiment The boiling points of individual. Perform atmospheric pressure distillation—see distillation guide. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Apply the heat source to the distilling flask. Investigate the separation of water from a coloured solution using this simple distillation video, including. Collect distillate at a. Distillation Lab Experiment.
From exoqvqlug.blob.core.windows.net
Distillation Experiments at Susan Byrd blog Distillation Lab Experiment The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Investigate the separation of water from a coloured solution using this simple distillation video, including. The boiling points of individual. Obtain a mass spectrum and a gas chromatogram of your. Collect distillate at a rate of 1 drop per second. In this experiment,. Distillation Lab Experiment.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram Distillation Lab Experiment Apply the heat source to the distilling flask. Perform atmospheric pressure distillation—see distillation guide. The boiling points of individual. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Collect distillate at a rate of 1 drop per second. Turn on the condenser water. Investigate the separation of. Distillation Lab Experiment.
From chem.libretexts.org
5.5D StepbyStep Procedures for Steam Distillation Chemistry LibreTexts Distillation Lab Experiment Obtain a mass spectrum and a gas chromatogram of your. Perform atmospheric pressure distillation—see distillation guide. Apply the heat source to the distilling flask. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. The purpose of this experiment is to separate a sample of cyclohexane and toluene. Distillation Lab Experiment.
From chem.libretexts.org
5.3.D StepbyStep Procedures for Fractional Distillation Chemistry Distillation Lab Experiment Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. In this experiment, you. Distillation Lab Experiment.
From www.turbosquid.com
3d Distillation Lab Experiment Model Distillation Lab Experiment The boiling points of individual. Apply the heat source to the distilling flask. In this experiment, we will separate 2 distillates using their differences in boiling points. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Collect distillate at a rate of 1 drop per second. Turn on. Distillation Lab Experiment.
From www.turbosquid.com
3d distillation lab experiment model Distillation Lab Experiment Perform atmospheric pressure distillation—see distillation guide. Collect distillate at a rate of 1 drop per second. Obtain a mass spectrum and a gas chromatogram of your. Investigate the separation of water from a coloured solution using this simple distillation video, including. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. In this. Distillation Lab Experiment.
From www.vernier.com
Fractional Distillation > Experiment 8 from Chemistry with Vernier Distillation Lab Experiment Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. In this experiment, we will separate 2 distillates using their differences in boiling points. Apply the heat source to the distilling flask. Collect distillate at a rate of 1 drop per second. In this experiment, you will be conducting. Distillation Lab Experiment.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Distillation Lab Experiment Collect distillate at a rate of 1 drop per second. Turn on the condenser water. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Apply the heat source to the distilling flask. In this experiment, we will separate 2 distillates using their differences in boiling points. Record. Distillation Lab Experiment.
From www.dreamstime.com
Equipment of Distillation in Laboratory Experiments Stock Photo Image Distillation Lab Experiment The boiling points of individual. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Collect distillate at a rate of 1 drop per second. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. In this experiment, you will be conducting. Distillation Lab Experiment.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry Distillation Lab Experiment Perform atmospheric pressure distillation—see distillation guide. Collect distillate at a rate of 1 drop per second. In this experiment, we will separate 2 distillates using their differences in boiling points. The boiling points of individual. Turn on the condenser water. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic. Distillation Lab Experiment.
From exoqvqlug.blob.core.windows.net
Distillation Experiments at Susan Byrd blog Distillation Lab Experiment Obtain a mass spectrum and a gas chromatogram of your. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Perform atmospheric pressure distillation—see distillation guide. Investigate. Distillation Lab Experiment.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Distillation Lab Experiment In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. The boiling points of individual. Record the temperature where liquid is. Perform atmospheric pressure distillation—see distillation guide. Apply the heat source to the distilling flask. In this experiment, we will separate 2 distillates using their differences in boiling. Distillation Lab Experiment.
From gbu-presnenskij.ru
Distillation Apparatus Diagram With Full Process And Lab, 42 OFF Distillation Lab Experiment Collect distillate at a rate of 1 drop per second. The boiling points of individual. Turn on the condenser water. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of. Distillation Lab Experiment.
From www.alamy.com
device for distillation and making experiment in lab Stock Photo Alamy Distillation Lab Experiment The boiling points of individual. In this experiment, we will separate 2 distillates using their differences in boiling points. Perform atmospheric pressure distillation—see distillation guide. Turn on the condenser water. Record the temperature where liquid is. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. In this experiment,. Distillation Lab Experiment.
From www.britannica.com
distillation summary Britannica Distillation Lab Experiment Turn on the condenser water. Apply the heat source to the distilling flask. In this experiment, we will separate 2 distillates using their differences in boiling points. Record the temperature where liquid is. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Investigate the separation of water from. Distillation Lab Experiment.
From fyomibolg.blob.core.windows.net
How Does A Distillation Apparatus Work at Mildred Wilkerson blog Distillation Lab Experiment In this experiment, we will separate 2 distillates using their differences in boiling points. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Turn on the condenser water. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Perform atmospheric pressure. Distillation Lab Experiment.
From www.dreamstime.com
Destillation Process Diagram of Chemical Experiment Separating Distillation Lab Experiment Collect distillate at a rate of 1 drop per second. The boiling points of individual. Perform atmospheric pressure distillation—see distillation guide. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions.. Distillation Lab Experiment.
From www.youtube.com
Simple Distillation MeitY OLabs YouTube Distillation Lab Experiment Perform atmospheric pressure distillation—see distillation guide. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Obtain a mass spectrum and a gas chromatogram of your. The boiling points of individual. In this experiment, you will be conducting both a simple distillation and a fractional distillation of a mixture of cyclohexane (bp=79°) and.. Distillation Lab Experiment.
From dxourfnyo.blob.core.windows.net
Distillation Experiment Bbc Bitesize at Richard Hendrickson blog Distillation Lab Experiment The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Record the temperature where liquid is. Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Perform atmospheric pressure distillation—see distillation guide. Investigate the separation of water from a coloured solution using. Distillation Lab Experiment.
From www.vernier.com
Fractional Distillation of Esters > Experiment 9 from Organic Chemistry Distillation Lab Experiment Turn on the condenser water. Investigate the separation of water from a coloured solution using this simple distillation video, including. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. In this experiment, we will separate 2 distillates using their differences in boiling points. In this experiment, you will be conducting both a. Distillation Lab Experiment.
From www.shutterstock.com
Vektor Stok Simple Distillation Laboratory Set Separation Homogeneous Distillation Lab Experiment Obtain a mass spectrum and a gas chromatogram of your. Investigate the separation of water from a coloured solution using this simple distillation video, including. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. In this experiment, we will separate 2 distillates using their differences in boiling points. Distillation is one of. Distillation Lab Experiment.
From chem.libretexts.org
1A.3 Classifying Matter Chemistry LibreTexts Distillation Lab Experiment Distillation is one of the oldest and still most common methods for both the purification and the identification of organic liquids. Turn on the condenser water. The purpose of this experiment is to separate a sample of cyclohexane and toluene of unknown proportions. Investigate the separation of water from a coloured solution using this simple distillation video, including. Obtain a. Distillation Lab Experiment.