Co Heating Value . 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the. Volumetric heating values are based on standard temperatures and pressure of dry. 1) nm3 = normal cubic metre. Water in the products is vapor. the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. the table below gives the gross and net heating value of fossil fuels as well as some alternative biobased fuels. 83 rows heating value. 1 btu/lb = 2,326.1 j/kg = 0.55556 kcal/kg. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. gross heating and net heating values for some common gases can be found in the table below: Water in the products is. there are two heating values:
from www.slideserve.com
84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the. Water in the products is vapor. Water in the products is. there are two heating values: The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. the table below gives the gross and net heating value of fossil fuels as well as some alternative biobased fuels. Volumetric heating values are based on standard temperatures and pressure of dry. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. gross heating and net heating values for some common gases can be found in the table below:
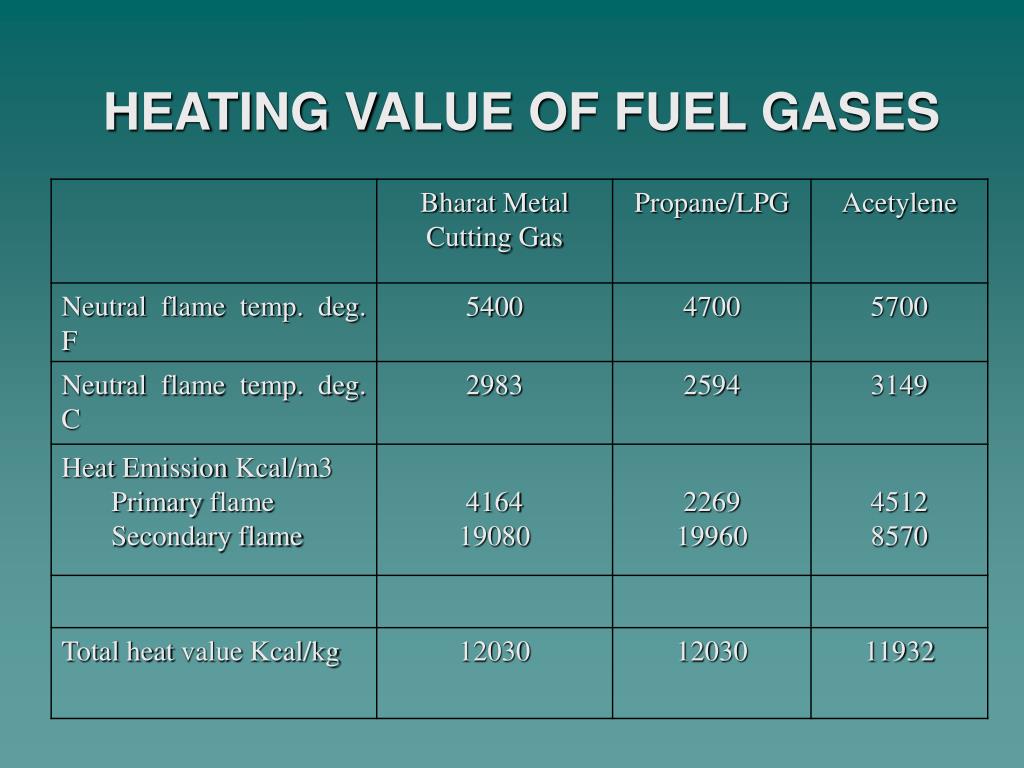
PPT BHARAT METAL CUTTING GAS PowerPoint Presentation, free download
Co Heating Value the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. gross heating and net heating values for some common gases can be found in the table below: the table below gives the gross and net heating value of fossil fuels as well as some alternative biobased fuels. Water in the products is vapor. 1 btu/lb = 2,326.1 j/kg = 0.55556 kcal/kg. the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. 83 rows heating value. Volumetric heating values are based on standard temperatures and pressure of dry. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. Water in the products is. there are two heating values: standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the. 1) nm3 = normal cubic metre.
From www.fixr.com
A Real Cost Comparison of Heating Options for Your Home Co Heating Value The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at. Co Heating Value.
From energyknowledgebase.com
Heating value · Energy KnowledgeBase Co Heating Value Water in the products is. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. Water in the products is vapor. 1) nm3 = normal cubic metre. 1 btu/lb = 2,326.1 j/kg = 0.55556 kcal/kg. gross heating and net heating values for some common gases. Co Heating Value.
From www.scribd.com
Average Heating Value Calorific Value Gross Energy Equiv alent Co Heating Value 83 rows heating value. the table below gives the gross and net heating value of fossil fuels as well as some alternative biobased fuels. Water in the products is. 1) nm3 = normal cubic metre. 1 btu/lb = 2,326.1 j/kg = 0.55556 kcal/kg. The heat of combustion for fuels is expressed as the hhv (higher heating value). Co Heating Value.
From www.slideserve.com
PPT Energy Measurement Concepts PowerPoint Presentation, free Co Heating Value the table below gives the gross and net heating value of fossil fuels as well as some alternative biobased fuels. Water in the products is. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. 83 rows heating value. there are two heating values: 1) nm3 = normal cubic metre.. Co Heating Value.
From www.researchgate.net
Maximum values of sensible heat (H), latent heat (LE), net ecosystem CO Co Heating Value there are two heating values: standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. Water in the products is vapor. 84 rows the heating value (or energy. Co Heating Value.
From www.animalia-life.club
Specific Heat Chart Of Common Substances Co Heating Value The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. Volumetric heating values are based on standard temperatures and pressure of dry. 1) nm3 = normal cubic metre. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. Water in. Co Heating Value.
From www.slideserve.com
PPT BHARAT METAL CUTTING GAS PowerPoint Presentation, free download Co Heating Value Volumetric heating values are based on standard temperatures and pressure of dry. Water in the products is vapor. gross heating and net heating values for some common gases can be found in the table below: standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. . Co Heating Value.
From www.intechopen.com
Table 1. Co Heating Value Volumetric heating values are based on standard temperatures and pressure of dry. 83 rows heating value. 1) nm3 = normal cubic metre. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. Water in the products is. The heat of combustion for fuels is expressed as. Co Heating Value.
From www.scribd.com
Lower and Higher Heating Values Energy Production Physical Chemistry Co Heating Value Water in the products is. gross heating and net heating values for some common gases can be found in the table below: there are two heating values: Volumetric heating values are based on standard temperatures and pressure of dry. the table below gives the gross and net heating value of fossil fuels as well as some alternative. Co Heating Value.
From www.grc.nasa.gov
Specific Heats Calorically Imperfect Gas Co Heating Value Water in the products is. Volumetric heating values are based on standard temperatures and pressure of dry. the table below gives the gross and net heating value of fossil fuels as well as some alternative biobased fuels. gross heating and net heating values for some common gases can be found in the table below: 84 rows the. Co Heating Value.
From mavink.com
Specific Heat Table Of Values Co Heating Value the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. 1) nm3 = normal cubic metre. Water in the products is. Volumetric heating values are based on standard temperatures and pressure of dry. standard enthalpies are precisely defined as the heat. Co Heating Value.
From www.nuclear-power.com
Overall Heat Transfer Coefficient Ufactor Definition nuclear Co Heating Value Volumetric heating values are based on standard temperatures and pressure of dry. 83 rows heating value. 1) nm3 = normal cubic metre. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. 1 btu/lb = 2,326.1 j/kg = 0.55556 kcal/kg. Water in the products is vapor. the table below gives. Co Heating Value.
From www.slideserve.com
PPT Lecture Objectives PowerPoint Presentation, free download ID Co Heating Value there are two heating values: Volumetric heating values are based on standard temperatures and pressure of dry. the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. 1) nm3 = normal cubic metre. 83 rows heating value. standard enthalpies. Co Heating Value.
From www.pharmacalculations.com
Overall Heat Transfer CoEfficient Calculation Pharma Engineering Co Heating Value The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. Volumetric heating values are based on standard temperatures and pressure of dry. the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. 1 btu/lb. Co Heating Value.
From energyknowledgebase.com
Heating value · Energy KnowledgeBase Co Heating Value The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. there are two heating values: Water in the products is vapor. the table below gives the gross and. Co Heating Value.
From www.researchgate.net
Heating value of several fuels. Download Table Co Heating Value Water in the products is vapor. Volumetric heating values are based on standard temperatures and pressure of dry. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. Water in the products is. 1) nm3 = normal cubic metre. the heat of combustion, also known as. Co Heating Value.
From www.semanticscholar.org
[PDF] Formulas for calculating the heating value of coal and coal char Co Heating Value there are two heating values: the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. Water in the products is. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see. Co Heating Value.
From www.slideserve.com
PPT Lecture 3. Fuel Cell Thermodynamics PowerPoint Presentation, free Co Heating Value the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. gross heating and net heating values for some common gases can be found in the table below: Water in the products is vapor. standard enthalpies are precisely defined as the. Co Heating Value.
From www.researchgate.net
The lower heating values and energy content of the unsorted solid waste Co Heating Value 1) nm3 = normal cubic metre. there are two heating values: 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give.. Co Heating Value.
From audran-frock.blogspot.com
Heating Value Ghv Gross Heating Value By Acronymsandslang Com Co Heating Value The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. 1) nm3 = normal cubic metre. Water in the products is vapor. gross heating. Co Heating Value.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID6948130 Co Heating Value 1 btu/lb = 2,326.1 j/kg = 0.55556 kcal/kg. the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. 83 rows heating value. . Co Heating Value.
From www.mdpi.com
Processes Free FullText Heat Transfer Coefficient Estimation and Co Heating Value standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. the table below gives the gross and net heating value of fossil fuels as well as some alternative biobased fuels. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv. Co Heating Value.
From www.powrmatic.co.uk
Calculating heat loss in a building. A guide to what you need to know Co Heating Value standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. Water in the products is vapor. gross heating and net heating values for some common gases can be found in the table below: Water in the products is. Volumetric heating values are based on standard temperatures. Co Heating Value.
From www.researchgate.net
6. Heat capacity of the flue gas depending on temperature and air Co Heating Value Volumetric heating values are based on standard temperatures and pressure of dry. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. 1) nm3 = normal cubic metre. Water in the products is. 83 rows heating value. there are two heating values: the heat of combustion, also known as the. Co Heating Value.
From inspectapedia.com
Definitions Heating, Cooling & Insulation Terms BTU, Calorie, R U& K Co Heating Value 1) nm3 = normal cubic metre. Water in the products is. Water in the products is vapor. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. . Co Heating Value.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation, free download ID1792465 Co Heating Value the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the. the table below gives the gross. Co Heating Value.
From www.researchgate.net
Fuel heating value to calculate furnace Watt power Download Table Co Heating Value standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. there are two heating values: Water in the products is vapor. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. Water in the products is. Volumetric heating values. Co Heating Value.
From www.eurovent.co.th
พลังงานความร้อนที่ได้จากเชื้อเพลิง…คือ Co Heating Value Water in the products is vapor. Volumetric heating values are based on standard temperatures and pressure of dry. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. Water in the products is. the heat of combustion, also known as the higher heating value, is a function of the number of moles. Co Heating Value.
From www.chegg.com
Some Heating Values Component HHV ( MJ/kg) LHV Co Heating Value the table below gives the gross and net heating value of fossil fuels as well as some alternative biobased fuels. Water in the products is vapor. 1 btu/lb = 2,326.1 j/kg = 0.55556 kcal/kg. gross heating and net heating values for some common gases can be found in the table below: Water in the products is. . Co Heating Value.
From audran-frock.blogspot.com
Heating Value Ghv Gross Heating Value By Acronymsandslang Com Co Heating Value the heat of combustion, also known as the higher heating value, is a function of the number of moles of water vapor formed and considering the heat. Volumetric heating values are based on standard temperatures and pressure of dry. there are two heating values: Water in the products is vapor. Water in the products is. the table. Co Heating Value.
From sitn.hms.harvard.edu
Climate Change 2016 Make America Hot Again Science in the News Co Heating Value Water in the products is vapor. standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. Water in the products is. 1 btu/lb = 2,326.1 j/kg = 0.55556 kcal/kg. 83 rows heating value. Volumetric heating values are based on standard temperatures and pressure of dry.. Co Heating Value.
From www.researchgate.net
Lower heating values of fuels and species involved in the EROI Co Heating Value gross heating and net heating values for some common gases can be found in the table below: Water in the products is vapor. 1) nm3 = normal cubic metre. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. Volumetric heating values are based on standard temperatures and pressure of dry. . Co Heating Value.
From energyknowledgebase.com
Heating value · Energy KnowledgeBase Co Heating Value 1 btu/lb = 2,326.1 j/kg = 0.55556 kcal/kg. 84 rows the heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the. Water in the products is vapor. 1) nm3 = normal cubic metre. The heat of combustion for fuels is expressed as the hhv (higher heating value). Co Heating Value.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6948711 Co Heating Value 83 rows heating value. the table below gives the gross and net heating value of fossil fuels as well as some alternative biobased fuels. Water in the products is. Water in the products is vapor. The heat of combustion for fuels is expressed as the hhv (higher heating value) or lhv (lower. 1) nm3 = normal cubic metre.. Co Heating Value.
From ketek.ca
Incineration Heating Value (HV) Ketek Group Co Heating Value there are two heating values: standard enthalpies are precisely defined as the heat energy absorbed or released when a process occurs at 25 o c to give. Volumetric heating values are based on standard temperatures and pressure of dry. Water in the products is. The heat of combustion for fuels is expressed as the hhv (higher heating value). Co Heating Value.