Lead Ground State Electron Configuration . Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. The ground state electron configuration of ground state gaseous. The orbital diagram is formed using hund’s rule and pauli exclusion principle. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. As 1s orbital is the. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. See the answer, explanation and. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. See examples for sodium, neon, silicon, and arsenic. Learn how to write and read electron configurations with examples and. Lead excited state electronic configuration ground state lead orbital diagram.
from www.slideserve.com
The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. See examples for sodium, neon, silicon, and arsenic. The orbital diagram is formed using hund’s rule and pauli exclusion principle. See the answer, explanation and. As 1s orbital is the. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. Learn how to write and read electron configurations with examples and. The ground state electron configuration of ground state gaseous.
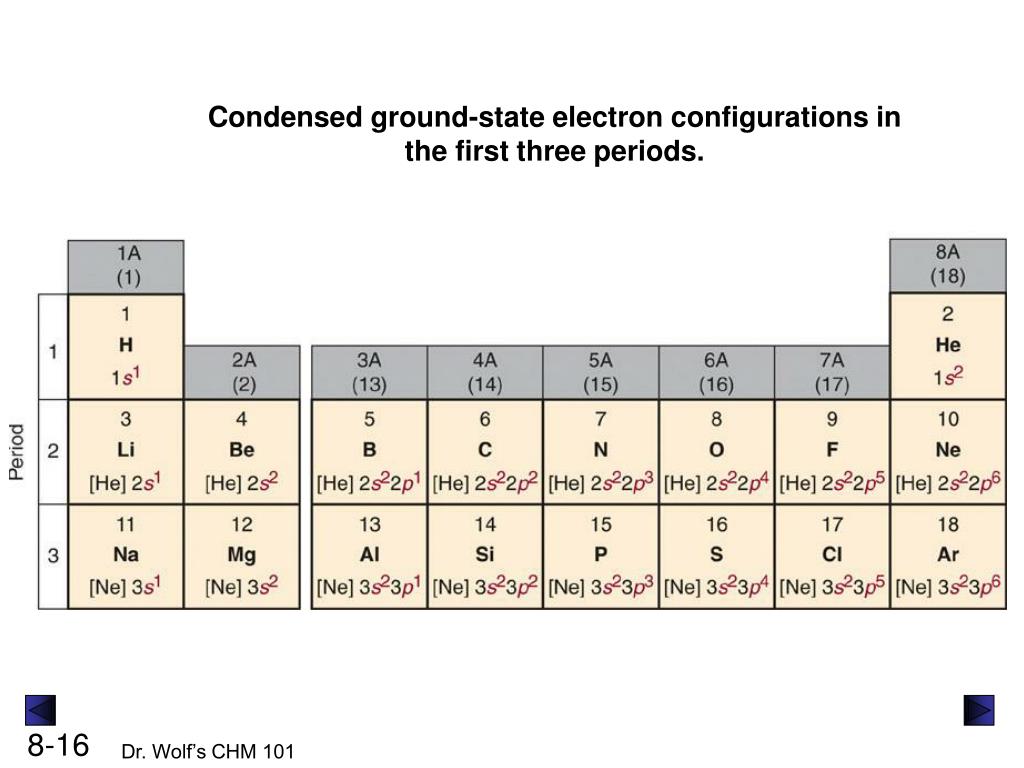
PPT Chapter 8 Electron Configuration and Chemical Periodicity
Lead Ground State Electron Configuration See examples for sodium, neon, silicon, and arsenic. Lead excited state electronic configuration ground state lead orbital diagram. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. Learn how to write and read electron configurations with examples and. As 1s orbital is the. See the answer, explanation and. The orbital diagram is formed using hund’s rule and pauli exclusion principle. The ground state electron configuration of ground state gaseous. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. See examples for sodium, neon, silicon, and arsenic. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels.
From solvedlib.com
The groundstate electron configuration contains thre… SolvedLib Lead Ground State Electron Configuration See examples for sodium, neon, silicon, and arsenic. Lead excited state electronic configuration ground state lead orbital diagram. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. Lead. Lead Ground State Electron Configuration.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Lead Ground State Electron Configuration The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. See the answer, explanation and. As 1s orbital is the. Learn how to write and read electron configurations with examples and. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. 119 rows find the electron configuration of carbon. Lead Ground State Electron Configuration.
From www.youtube.com
Electron Configuration of Lead Pb Lesson YouTube Lead Ground State Electron Configuration The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. As 1s orbital is the. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. See the answer,. Lead Ground State Electron Configuration.
From www.slideserve.com
PPT Chapter 8 Electron Configuration and Chemical Periodicity Lead Ground State Electron Configuration Learn how to write and read electron configurations with examples and. As 1s orbital is the. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. Lead excited state. Lead Ground State Electron Configuration.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Lead Ground State Electron Configuration The orbital diagram is formed using hund’s rule and pauli exclusion principle. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. See examples for sodium, neon, silicon, and arsenic. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. The ground state electron configuration of ground state gaseous.. Lead Ground State Electron Configuration.
From www.researchgate.net
Electronic configurations energy levels of the ground state 3 O 2 Lead Ground State Electron Configuration The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. The ground state electron configuration of ground state gaseous. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. Lead excited state electronic configuration ground state lead orbital diagram.. Lead Ground State Electron Configuration.
From quizlet.com
What is the ground state electron configuration and orbital Quizlet Lead Ground State Electron Configuration The orbital diagram is formed using hund’s rule and pauli exclusion principle. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. See examples for sodium, neon, silicon, and arsenic. See the answer, explanation and. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. 119 rows find. Lead Ground State Electron Configuration.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Lead Ground State Electron Configuration The ground state electron configuration of ground state gaseous. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. See examples for sodium, neon, silicon, and arsenic. Lead excited state electronic configuration ground state lead orbital diagram. Learn how to write and read electron configurations with examples and. The. Lead Ground State Electron Configuration.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Lead Ground State Electron Configuration 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. The ground state electron configuration of ground state gaseous. As 1s orbital is the. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The orbital diagram is formed using hund’s rule and pauli exclusion principle. Learn the. Lead Ground State Electron Configuration.
From study.com
Ground State Electron Configuration of an Atom Rules, Terms Lead Ground State Electron Configuration 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. As 1s orbital is the. Lead excited state electronic configuration ground state lead orbital diagram. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. The ground state. Lead Ground State Electron Configuration.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Lead Ground State Electron Configuration As 1s orbital is the. Lead excited state electronic configuration ground state lead orbital diagram. The orbital diagram is formed using hund’s rule and pauli exclusion principle. See the answer, explanation and. See examples for sodium, neon, silicon, and arsenic. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The electron configuration of lead (pb) is described by. Lead Ground State Electron Configuration.
From www.youtube.com
CHEMISTRY 101 Writing an Electron Configuration for Lead Using the Lead Ground State Electron Configuration See the answer, explanation and. The ground state electron configuration of ground state gaseous. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. The orbital diagram is formed using hund’s rule and pauli exclusion principle. Learn how to write and read electron configurations with examples and. The electron. Lead Ground State Electron Configuration.
From knowledgebin.org
Atomic Structure7 Ground state electron configuration for chromium Lead Ground State Electron Configuration The orbital diagram is formed using hund’s rule and pauli exclusion principle. See the answer, explanation and. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. See examples for sodium, neon, silicon, and arsenic. 119 rows find the electron configuration of carbon (c) and other elements in a table. Lead Ground State Electron Configuration.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Ground State Electron Configuration 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. As 1s orbital is the. The orbital diagram is formed using hund’s rule and pauli exclusion principle. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Lead excited state electronic configuration ground state lead orbital diagram. The. Lead Ground State Electron Configuration.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Lead Ground State Electron Configuration Learn how to write and read electron configurations with examples and. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Lead excited state electronic configuration ground state lead orbital diagram. See examples for sodium, neon, silicon, and arsenic. As 1s orbital is the. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons. Lead Ground State Electron Configuration.
From iperiodictable.com
Ground State Electron Configuration of V Periodic Table Element Lead Ground State Electron Configuration See the answer, explanation and. The orbital diagram is formed using hund’s rule and pauli exclusion principle. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. Learn the. Lead Ground State Electron Configuration.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Lead Ground State Electron Configuration Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. See the answer, explanation and. See examples for sodium, neon, silicon, and arsenic. Learn how to write and. Lead Ground State Electron Configuration.
From iperiodictable.com
How To Find an Valence Lead Electron Configuration (Pb) Lead Ground State Electron Configuration The orbital diagram is formed using hund’s rule and pauli exclusion principle. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. See examples for sodium, neon, silicon, and arsenic. See the answer, explanation and. 119 rows find the electron configuration of carbon (c) and other elements in a. Lead Ground State Electron Configuration.
From socratic.org
Write the ground state electron configuration for a neutral carbon atom Lead Ground State Electron Configuration The ground state electron configuration of ground state gaseous. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. Lead atoms have 82 electrons and the shell structure is. Lead Ground State Electron Configuration.
From sciencenotes.org
List of Electron Configurations of Elements Lead Ground State Electron Configuration Lead excited state electronic configuration ground state lead orbital diagram. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. The orbital diagram is formed using hund’s rule and. Lead Ground State Electron Configuration.
From brandonkss.github.io
Ground State Electron Configuration Chart Lead Ground State Electron Configuration 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. The electron configuration of lead (pb) is. Lead Ground State Electron Configuration.
From www.sliderbase.com
Groundstate electron configurations Lead Ground State Electron Configuration See examples for sodium, neon, silicon, and arsenic. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. The orbital diagram is formed using hund’s rule and pauli exclusion principle. As 1s orbital is the. Lead excited state electronic configuration ground state lead orbital diagram. 119 rows find the electron. Lead Ground State Electron Configuration.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Lead Ground State Electron Configuration Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The orbital diagram is formed using hund’s rule and pauli exclusion principle. As 1s orbital is the. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. The electron configuration of lead (pb) is described by its atomic. Lead Ground State Electron Configuration.
From www.slideserve.com
PPT CHAPTER 5 PowerPoint Presentation, free download ID5502851 Lead Ground State Electron Configuration The ground state electron configuration of ground state gaseous. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. See examples for sodium, neon, silicon, and arsenic. Lead. Lead Ground State Electron Configuration.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Ground State Electron Configuration Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. See examples for sodium, neon, silicon, and arsenic. Learn how to write and read electron configurations with examples and. See the answer, explanation and. The orbital diagram is formed. Lead Ground State Electron Configuration.
From studylib.net
Ground State Electron Configurations • The actual wavefunction of a Lead Ground State Electron Configuration Lead excited state electronic configuration ground state lead orbital diagram. As 1s orbital is the. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. See examples for sodium, neon, silicon, and arsenic. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. Learn how to write and read. Lead Ground State Electron Configuration.
From mungfali.com
Ground State Electron Configuration Chart Lead Ground State Electron Configuration The orbital diagram is formed using hund’s rule and pauli exclusion principle. Lead excited state electronic configuration ground state lead orbital diagram. See examples for sodium, neon, silicon, and arsenic. The ground state electron configuration of ground state gaseous. See the answer, explanation and. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed. Lead Ground State Electron Configuration.
From www.orangatame.com
How to Write Ground State Electron Configuration in Chemistry Lead Ground State Electron Configuration See examples for sodium, neon, silicon, and arsenic. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. Lead excited state electronic configuration ground state lead orbital diagram. Learn how to write and read electron configurations with examples and. The ground state electron configuration of ground state gaseous. 119. Lead Ground State Electron Configuration.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead Ground State Electron Configuration Lead excited state electronic configuration ground state lead orbital diagram. See the answer, explanation and. The orbital diagram is formed using hund’s rule and pauli exclusion principle. Learn how to write and read electron configurations with examples and. As 1s orbital is the. Learn the basic steps and methods to write the electron configuration of an atom or ion in. Lead Ground State Electron Configuration.
From brandonkss.github.io
Ground State Electron Configuration Chart Lead Ground State Electron Configuration See examples for sodium, neon, silicon, and arsenic. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. See the answer, explanation and. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. 119 rows find the electron configuration. Lead Ground State Electron Configuration.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Lead Ground State Electron Configuration The ground state electron configuration of ground state gaseous. See examples for sodium, neon, silicon, and arsenic. Learn how to write and read electron configurations with examples and. The orbital diagram is formed using hund’s rule and pauli exclusion principle. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The electron configuration of lead (pb) is described by. Lead Ground State Electron Configuration.
From firsteducationinfo.com
Ground State Electron Configuration First Education Info Lead Ground State Electron Configuration Lead excited state electronic configuration ground state lead orbital diagram. Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The ground state electron configuration of ground state gaseous. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. Learn the basic steps and methods to write the. Lead Ground State Electron Configuration.
From examquiz.netlify.app
What is ground state electron configuration examquiz Lead Ground State Electron Configuration The orbital diagram is formed using hund’s rule and pauli exclusion principle. See the answer, explanation and. See examples for sodium, neon, silicon, and arsenic. As 1s orbital is the. 119 rows find the electron configuration of carbon (c) and other elements in a table with shorthand, full and shell notation. Learn the basic steps and methods to write the. Lead Ground State Electron Configuration.
From geteducationskills.com
Ground State Electron Configuration An Overview Get Education Lead Ground State Electron Configuration See the answer, explanation and. The orbital diagram is formed using hund’s rule and pauli exclusion principle. The ground state electron configuration of ground state gaseous. As 1s orbital is the. Learn the basic steps and methods to write the electron configuration of an atom or ion in its lowest energy state. The electron configuration of lead (pb) is described. Lead Ground State Electron Configuration.
From www.slideserve.com
PPT Ground State Electron Configurations and Term Symbols PowerPoint Lead Ground State Electron Configuration See examples for sodium, neon, silicon, and arsenic. The orbital diagram is formed using hund’s rule and pauli exclusion principle. Learn how to write and read electron configurations with examples and. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy levels. See the answer, explanation and. The ground state electron. Lead Ground State Electron Configuration.