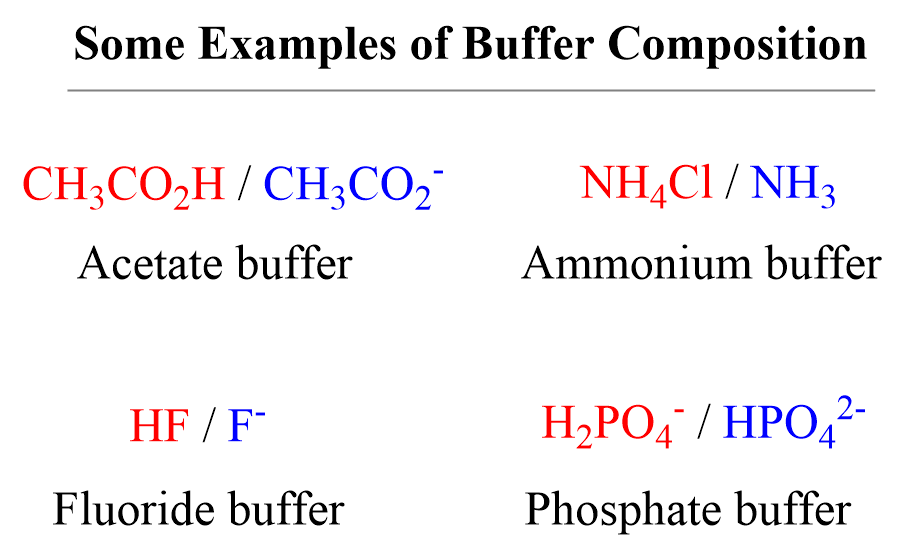
What Is The Purpose Of A Buffer Solution . buffer solutions play a large role in biochemical functions. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. Because buffers resist changes in ph levels, they. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is.
from www.pinterest.es
a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. Because buffers resist changes in ph levels, they. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. buffer solutions play a large role in biochemical functions. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
Understanding Buffer Solution Chemistry A Complete Guide
What Is The Purpose Of A Buffer Solution a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. buffer solutions play a large role in biochemical functions. Because buffers resist changes in ph levels, they. the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and.
From www.slideshare.net
Buffer system What Is The Purpose Of A Buffer Solution a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. Because buffers resist changes in ph levels, they. . What Is The Purpose Of A Buffer Solution.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Is The Purpose Of A Buffer Solution the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Because buffers resist changes in ph levels, they. buffer solutions are one of the most important. What Is The Purpose Of A Buffer Solution.
From www.tes.com
Buffer Solutions 3 (A Level Chemistry) Teaching Resources What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. Because buffers resist changes in ph levels, they. a buffer is an aqueous solution that consists. What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Hydrolysis of Salts and pH of Buffer Solutions PowerPoint Presentation ID5498761 What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. Because buffers resist changes in ph levels, they. a solution whose ph is. What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID4273107 What Is The Purpose Of A Buffer Solution a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. Because buffers resist changes in ph levels, they. . What Is The Purpose Of A Buffer Solution.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Experiment GoldBio What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. buffer solutions play a large role in biochemical functions. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a solution whose ph is not altered to any. What Is The Purpose Of A Buffer Solution.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Is The Purpose Of A Buffer Solution Because buffers resist changes in ph levels, they. buffer solutions play a large role in biochemical functions. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. buffer solutions are one of the most important types of chemical reagent used in chemical. What Is The Purpose Of A Buffer Solution.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. the purpose of a buffer solution is to maintain a relatively constant ph,. What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Is The Purpose Of A Buffer Solution a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Because buffers resist changes in ph levels, they. . What Is The Purpose Of A Buffer Solution.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is The Purpose Of A Buffer Solution a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a solution whose ph is not altered to any great extent by the. What Is The Purpose Of A Buffer Solution.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog What Is The Purpose Of A Buffer Solution the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a solution whose ph is not altered to any great extent by the addition of small. What Is The Purpose Of A Buffer Solution.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. the purpose of a buffer solution is to maintain a relatively constant ph, even. What Is The Purpose Of A Buffer Solution.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its action. YouTube What Is The Purpose Of A Buffer Solution a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffer solutions play a large role in biochemical functions. buffer solutions are one. What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Buffer Solutions, Function PowerPoint Presentation, free download ID4789528 What Is The Purpose Of A Buffer Solution a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. Because buffers resist changes in ph levels, they. . What Is The Purpose Of A Buffer Solution.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Because buffers resist changes in ph levels, they. a buffer is a solution that. What Is The Purpose Of A Buffer Solution.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions What Is The Purpose Of A Buffer Solution the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. buffer solutions play a large role in biochemical functions. Because buffers resist changes in ph levels, they. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research. What Is The Purpose Of A Buffer Solution.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution What Is The Purpose Of A Buffer Solution a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. the purpose of a buffer solution is to maintain a relatively constant ph,. What Is The Purpose Of A Buffer Solution.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is The Purpose Of A Buffer Solution buffer solutions play a large role in biochemical functions. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. a buffer is an aqueous solution. What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 What Is The Purpose Of A Buffer Solution the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffer solutions are one of the most important types of chemical reagent used in chemical research,. What Is The Purpose Of A Buffer Solution.
From fr.slideshare.net
Buffer solution What Is The Purpose Of A Buffer Solution the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. Because buffers resist changes in ph levels, they. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. buffer solutions play. What Is The Purpose Of A Buffer Solution.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Is The Purpose Of A Buffer Solution buffer solutions play a large role in biochemical functions. Because buffers resist changes in ph levels, they. the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research. What Is The Purpose Of A Buffer Solution.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation What Is The Purpose Of A Buffer Solution buffer solutions play a large role in biochemical functions. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. Because buffers resist changes. What Is The Purpose Of A Buffer Solution.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Because buffers resist changes in ph levels, they. the purpose of a buffer solution is to maintain a relatively. What Is The Purpose Of A Buffer Solution.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide What Is The Purpose Of A Buffer Solution a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that can resist. What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Is The Purpose Of A Buffer Solution a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Because buffers resist changes in ph levels, they. . What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download ID5348308 What Is The Purpose Of A Buffer Solution Because buffers resist changes in ph levels, they. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. a solution whose ph is. What Is The Purpose Of A Buffer Solution.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Is The Purpose Of A Buffer Solution the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. buffer solutions play a large role in biochemical functions. a buffer is a solution that. What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Presentation ID4395396 What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. buffer solutions play a large role in biochemical functions. a buffer is. What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5845696 What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. buffer solutions play a large role in biochemical functions. Because buffers resist changes in ph levels, they. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid. What Is The Purpose Of A Buffer Solution.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download What Is The Purpose Of A Buffer Solution the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. Because buffers resist changes in ph levels, they. a buffer is an aqueous solution that consists. What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504 What Is The Purpose Of A Buffer Solution Because buffers resist changes in ph levels, they. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid buffer) or a weak base with. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. . What Is The Purpose Of A Buffer Solution.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. a buffer is a solution that can resist ph change upon the addition of. What Is The Purpose Of A Buffer Solution.
From www.youtube.com
What is Buffer Solution Types of Buffer Solution Acidic Buffer and Basic Buffer YouTube What Is The Purpose Of A Buffer Solution buffer solutions are one of the most important types of chemical reagent used in chemical research, biological research and. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. a buffer is an aqueous solution that consists of a mixture of a weak acid and its salt (acid. What Is The Purpose Of A Buffer Solution.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses What Is The Purpose Of A Buffer Solution the purpose of a buffer solution is to maintain a relatively constant ph, even when small amounts of acids or bases are. a solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Because buffers resist changes in ph levels, they. a buffer is. What Is The Purpose Of A Buffer Solution.