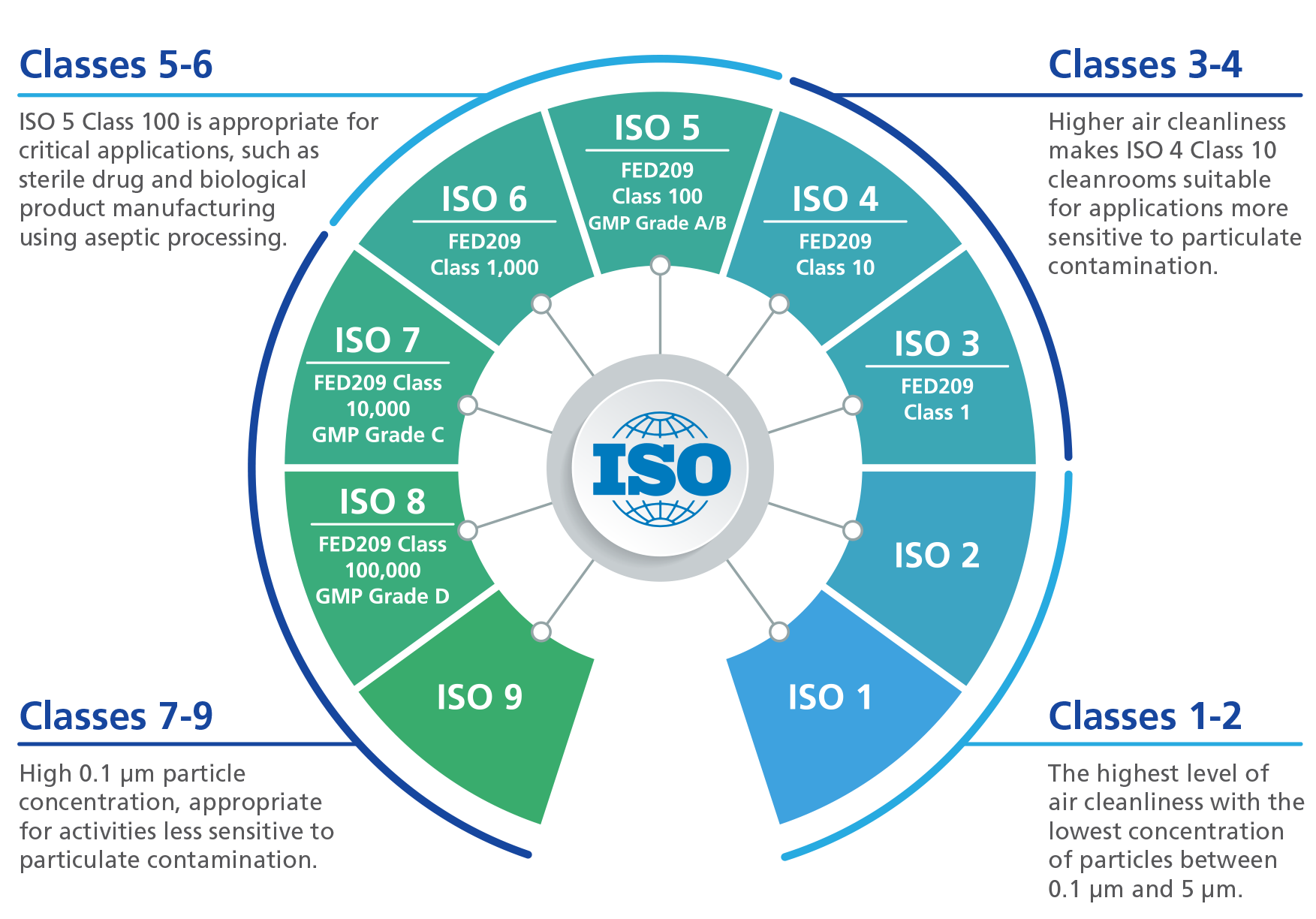
From dxouudmtz.blob.core.windows.net
Who Cleanroom Classification at Bethany Belt blog Who Gmp Clean Room Classification All cleanrooms are classified according. Understanding grade a, grade b, grade c & d. Cleanroom classifications and particles count. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations. By definition, cleanrooms are classified. Who Gmp Clean Room Classification.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Who Gmp Clean Room Classification By definition, cleanrooms are classified. who good practices for pharmaceutical microbiology laboratories. Understanding grade a, grade b, grade c & d. All cleanrooms are classified according. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Cleanroom classifications and particles count. The who expert committee on specifi cations. Who Gmp Clean Room Classification.
From www.iqsdirectory.com
Cleanroom What is it? ISO Standards and Classifications, Design, Types, Construction Who Gmp Clean Room Classification The who expert committee on specifi cations. Cleanroom classifications and particles count. All cleanrooms are classified according. who good practices for pharmaceutical microbiology laboratories. Understanding grade a, grade b, grade c & d. By definition, cleanrooms are classified. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Who Gmp Clean Room Classification.
From oms-technology.com
Pharmaceutical GMP Cleanroom Biosafety laboratory design & installation services Malaysia Who Gmp Clean Room Classification By definition, cleanrooms are classified. who good practices for pharmaceutical microbiology laboratories. Cleanroom classifications and particles count. All cleanrooms are classified according. Understanding grade a, grade b, grade c & d. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. The who expert committee on specifi cations. Who Gmp Clean Room Classification.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Gmp Clean Room Classification By definition, cleanrooms are classified. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Cleanroom classifications and particles count. The who expert committee on specifi cations. All cleanrooms are classified according. who good practices for pharmaceutical microbiology laboratories. Understanding grade a, grade b, grade c & d. Who Gmp Clean Room Classification.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Who Gmp Clean Room Classification Understanding grade a, grade b, grade c & d. By definition, cleanrooms are classified. Cleanroom classifications and particles count. who good practices for pharmaceutical microbiology laboratories. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. All cleanrooms are classified according. The who expert committee on specifi cations. Who Gmp Clean Room Classification.
From mavink.com
Cleanroom Classification Chart Who Gmp Clean Room Classification who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Understanding grade a, grade b, grade c & d. All cleanrooms are classified according. Cleanroom classifications and particles count. By definition, cleanrooms are classified. Who Gmp Clean Room Classification.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Who Gmp Clean Room Classification Cleanroom classifications and particles count. Understanding grade a, grade b, grade c & d. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. who good practices for pharmaceutical microbiology laboratories. All cleanrooms are classified according. The who expert committee on specifi cations. By definition, cleanrooms are classified. Who Gmp Clean Room Classification.
From schillingengineering.de
Cleanroom classes according to ISO 146441 and GMP Who Gmp Clean Room Classification Understanding grade a, grade b, grade c & d. All cleanrooms are classified according. By definition, cleanrooms are classified. who good practices for pharmaceutical microbiology laboratories. Cleanroom classifications and particles count. The who expert committee on specifi cations. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Who Gmp Clean Room Classification.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Who Gmp Clean Room Classification All cleanrooms are classified according. By definition, cleanrooms are classified. The who expert committee on specifi cations. Understanding grade a, grade b, grade c & d. who good practices for pharmaceutical microbiology laboratories. Cleanroom classifications and particles count. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Who Gmp Clean Room Classification.
From www.mecart-cleanrooms.com
Building a GMP Facility 8 GMP Cleanroom Requirements MECART Who Gmp Clean Room Classification All cleanrooms are classified according. The who expert committee on specifi cations. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Cleanroom classifications and particles count. By definition, cleanrooms are classified. Understanding grade a, grade b, grade c & d. who good practices for pharmaceutical microbiology laboratories. Who Gmp Clean Room Classification.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Who Gmp Clean Room Classification All cleanrooms are classified according. Cleanroom classifications and particles count. The who expert committee on specifi cations. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. By definition, cleanrooms are classified. Understanding grade a, grade b, grade c & d. who good practices for pharmaceutical microbiology laboratories. Who Gmp Clean Room Classification.
From www.aroundlabnews.com
AROUND LAB NEWS / IT » Understanding Cleanroom Classifications Who Gmp Clean Room Classification Cleanroom classifications and particles count. The who expert committee on specifi cations. who good practices for pharmaceutical microbiology laboratories. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Understanding grade a, grade b, grade c & d. All cleanrooms are classified according. By definition, cleanrooms are classified. Who Gmp Clean Room Classification.
From www.beckman.com
GMP Cleanrooms Classification and Routine Environmental Monitoring Who Gmp Clean Room Classification The who expert committee on specifi cations. who good practices for pharmaceutical microbiology laboratories. By definition, cleanrooms are classified. All cleanrooms are classified according. Cleanroom classifications and particles count. Understanding grade a, grade b, grade c & d. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Who Gmp Clean Room Classification.
From www.golighthouse.com
Cleanroom Classifications Explained Lighthouse Worldwide Solutions Who Gmp Clean Room Classification By definition, cleanrooms are classified. The who expert committee on specifi cations. who good practices for pharmaceutical microbiology laboratories. Cleanroom classifications and particles count. Understanding grade a, grade b, grade c & d. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. All cleanrooms are classified according. Who Gmp Clean Room Classification.
From www.semanticscholar.org
Table 1 from Pharmaceutical Cleanroom Classification using ISO 146441 and the EU GGMP Annex 1 Who Gmp Clean Room Classification Cleanroom classifications and particles count. By definition, cleanrooms are classified. who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations. Understanding grade a, grade b, grade c & d. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. All cleanrooms are classified according. Who Gmp Clean Room Classification.
From us.ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Gmp Clean Room Classification The who expert committee on specifi cations. Understanding grade a, grade b, grade c & d. All cleanrooms are classified according. who good practices for pharmaceutical microbiology laboratories. By definition, cleanrooms are classified. Cleanroom classifications and particles count. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Who Gmp Clean Room Classification.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Gmp Clean Room Classification By definition, cleanrooms are classified. The who expert committee on specifi cations. who good practices for pharmaceutical microbiology laboratories. All cleanrooms are classified according. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Understanding grade a, grade b, grade c & d. Cleanroom classifications and particles count. Who Gmp Clean Room Classification.
From yichunjh.en.made-in-china.com
GMP Standard Design Clean Room Classification Gain Good Reputation China Clean Room Project Who Gmp Clean Room Classification All cleanrooms are classified according. who good practices for pharmaceutical microbiology laboratories. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Understanding grade a, grade b, grade c & d. By definition, cleanrooms are classified. Cleanroom classifications and particles count. The who expert committee on specifi cations. Who Gmp Clean Room Classification.
From ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Gmp Clean Room Classification By definition, cleanrooms are classified. Cleanroom classifications and particles count. The who expert committee on specifi cations. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. who good practices for pharmaceutical microbiology laboratories. Understanding grade a, grade b, grade c & d. All cleanrooms are classified according. Who Gmp Clean Room Classification.
From berkshire.com
The Fundamentals of GMP Cleanroom Classifications Berkshire Corporation Who Gmp Clean Room Classification the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Understanding grade a, grade b, grade c & d. who good practices for pharmaceutical microbiology laboratories. By definition, cleanrooms are classified. Cleanroom classifications and particles count. All cleanrooms are classified according. The who expert committee on specifi cations. Who Gmp Clean Room Classification.
From www.testotis.com
Worth knowing from the area of cleanroom qualification Who Gmp Clean Room Classification All cleanrooms are classified according. The who expert committee on specifi cations. Cleanroom classifications and particles count. who good practices for pharmaceutical microbiology laboratories. Understanding grade a, grade b, grade c & d. By definition, cleanrooms are classified. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Who Gmp Clean Room Classification.
From www.cleanroom-industries.com
GMP in Cleanroom Maintenance Who Gmp Clean Room Classification By definition, cleanrooms are classified. Understanding grade a, grade b, grade c & d. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. All cleanrooms are classified according. Cleanroom classifications and particles count. The who expert committee on specifi cations. who good practices for pharmaceutical microbiology laboratories. Who Gmp Clean Room Classification.
From us.ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Gmp Clean Room Classification the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. All cleanrooms are classified according. Understanding grade a, grade b, grade c & d. who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations. By definition, cleanrooms are classified. Cleanroom classifications and particles count. Who Gmp Clean Room Classification.
From us.ngscleanrooms.com
Cleanroom Classification ISO 14644 FED STD 209 GMP Annex Who Gmp Clean Room Classification Understanding grade a, grade b, grade c & d. The who expert committee on specifi cations. By definition, cleanrooms are classified. All cleanrooms are classified according. who good practices for pharmaceutical microbiology laboratories. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Cleanroom classifications and particles count. Who Gmp Clean Room Classification.
From gmplabeling.com
Demystifying Cleanrooms Understanding Their Importance, Function, and Classification Standards Who Gmp Clean Room Classification All cleanrooms are classified according. Cleanroom classifications and particles count. who good practices for pharmaceutical microbiology laboratories. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Understanding grade a, grade b, grade c & d. The who expert committee on specifi cations. By definition, cleanrooms are classified. Who Gmp Clean Room Classification.
From www.phchd.com
ISO Certification and GMP Cleanroom Standards PHCbi Who Gmp Clean Room Classification who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. By definition, cleanrooms are classified. Cleanroom classifications and particles count. Understanding grade a, grade b, grade c & d. All cleanrooms are classified according. Who Gmp Clean Room Classification.
From berkshire.com
The Fundamentals of GMP Cleanroom Classifications Berkshire Corporation Who Gmp Clean Room Classification Cleanroom classifications and particles count. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. By definition, cleanrooms are classified. The who expert committee on specifi cations. All cleanrooms are classified according. who good practices for pharmaceutical microbiology laboratories. Understanding grade a, grade b, grade c & d. Who Gmp Clean Room Classification.
From www.golighthouse.com
GMP Annex 1 2022 Update Breakdown Part 1 Lighthouse Worldwide Solutions Who Gmp Clean Room Classification Understanding grade a, grade b, grade c & d. Cleanroom classifications and particles count. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. The who expert committee on specifi cations. All cleanrooms are classified according. By definition, cleanrooms are classified. who good practices for pharmaceutical microbiology laboratories. Who Gmp Clean Room Classification.
From www.presentationeze.com
Cleanroom Classification ISO 14644PresentationEZE Who Gmp Clean Room Classification Cleanroom classifications and particles count. Understanding grade a, grade b, grade c & d. All cleanrooms are classified according. By definition, cleanrooms are classified. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations. Who Gmp Clean Room Classification.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Who Gmp Clean Room Classification All cleanrooms are classified according. who good practices for pharmaceutical microbiology laboratories. The who expert committee on specifi cations. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Cleanroom classifications and particles count. By definition, cleanrooms are classified. Understanding grade a, grade b, grade c & d. Who Gmp Clean Room Classification.
From dxouudmtz.blob.core.windows.net
Who Cleanroom Classification at Bethany Belt blog Who Gmp Clean Room Classification All cleanrooms are classified according. Understanding grade a, grade b, grade c & d. The who expert committee on specifi cations. By definition, cleanrooms are classified. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Cleanroom classifications and particles count. who good practices for pharmaceutical microbiology laboratories. Who Gmp Clean Room Classification.
From pharmastate.academy
Cleanroom Classifications, Classes and ISO Standards Who Gmp Clean Room Classification Understanding grade a, grade b, grade c & d. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. By definition, cleanrooms are classified. All cleanrooms are classified according. The who expert committee on specifi cations. Cleanroom classifications and particles count. who good practices for pharmaceutical microbiology laboratories. Who Gmp Clean Room Classification.
From angstromtechnology.com
Cleanroom Classifications & Standards Angstrom Technology Who Gmp Clean Room Classification By definition, cleanrooms are classified. who good practices for pharmaceutical microbiology laboratories. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. All cleanrooms are classified according. The who expert committee on specifi cations. Cleanroom classifications and particles count. Understanding grade a, grade b, grade c & d. Who Gmp Clean Room Classification.
From gmpinsiders.com
GMP Cleanroom Classifications Understand Class A, B, C And D Who Gmp Clean Room Classification Cleanroom classifications and particles count. The who expert committee on specifi cations. By definition, cleanrooms are classified. All cleanrooms are classified according. who good practices for pharmaceutical microbiology laboratories. the gmp clean air grades and classifications define the environment in which sterile drugs and biological products should be manufactured. Understanding grade a, grade b, grade c & d. Who Gmp Clean Room Classification.