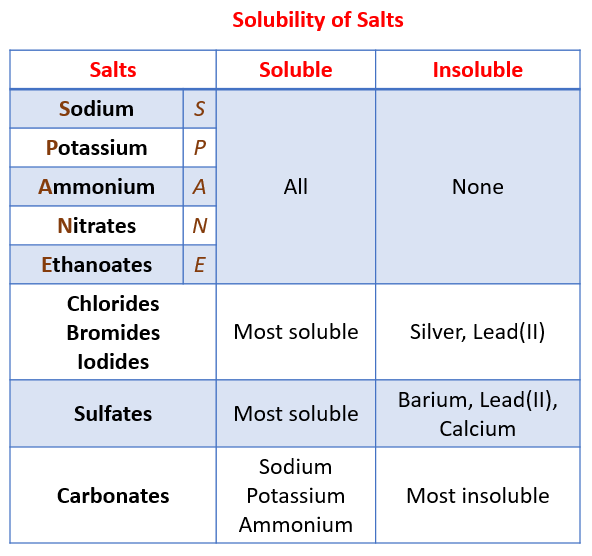
Is Ammonia An Acid Base Or Salt . Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. In short, nh3, ammonia, it is considered a base but it is a weak base. Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. This is a simple example of ammonia reacting with an acid to make a salt. Note that ammonia forms ammonium salts when it reacts with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. For instance, ammonia reacts with hydrochloric acid to make ammonium. Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. As explained earlier, nh3 is a weak base and reacts with acids to form salts. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. Ammonia doesn't just dissolve in water; Nh3 (ammonia) is one of.
from www.onlinemathlearning.com
Nh3 (ammonia) is one of. In short, nh3, ammonia, it is considered a base but it is a weak base. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Note that ammonia forms ammonium salts when it reacts with acids. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. As explained earlier, nh3 is a weak base and reacts with acids to form salts. This is a simple example of ammonia reacting with an acid to make a salt. Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. For instance, ammonia reacts with hydrochloric acid to make ammonium.
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets
Is Ammonia An Acid Base Or Salt In short, nh3, ammonia, it is considered a base but it is a weak base. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. Ammonia doesn't just dissolve in water; For instance, ammonia reacts with hydrochloric acid to make ammonium. In short, nh3, ammonia, it is considered a base but it is a weak base. Note that ammonia forms ammonium salts when it reacts with acids. Nh3 (ammonia) is one of. Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. This is a simple example of ammonia reacting with an acid to make a salt. As explained earlier, nh3 is a weak base and reacts with acids to form salts. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Is Ammonia An Acid Base Or Salt This is a simple example of ammonia reacting with an acid to make a salt. In short, nh3, ammonia, it is considered a base but it is a weak base. Note that ammonia forms ammonium salts when it reacts with acids. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with.. Is Ammonia An Acid Base Or Salt.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Salts Chemistry Revision Notes & Tests Is Ammonia An Acid Base Or Salt This is a simple example of ammonia reacting with an acid to make a salt. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Ammonia doesn't just dissolve in water; Although nh3 is a weak base, it also acts as a weak acid under certain conditions and. Is Ammonia An Acid Base Or Salt.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Is Ammonia An Acid Base Or Salt Ammonia doesn't just dissolve in water; Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. This is a simple example of ammonia reacting with an. Is Ammonia An Acid Base Or Salt.
From www.slideserve.com
PPT “ Acids, Bases, and Salts ” PowerPoint Presentation, free Is Ammonia An Acid Base Or Salt Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts. Is Ammonia An Acid Base Or Salt.
From www.slideserve.com
PPT To understand two models of acids and bases PowerPoint Is Ammonia An Acid Base Or Salt For instance, ammonia reacts with hydrochloric acid to make ammonium. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. In short, nh3, ammonia, it is considered a base but it is a weak base. This is a simple example of ammonia reacting with an acid to make. Is Ammonia An Acid Base Or Salt.
From www.sliderbase.com
Acid, bases and salts Presentation Chemistry Is Ammonia An Acid Base Or Salt Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. Nh3 (ammonia) is one of. Ammonia doesn't just dissolve in water; This is a simple example of ammonia reacting with an acid to make a salt. Although nh3 is a weak base, it also acts as a weak. Is Ammonia An Acid Base Or Salt.
From www.chemicals.co.uk
The Surprising Reaction Of Nitric Acid & Ammonia The Chemistry Blog Is Ammonia An Acid Base Or Salt Ammonia doesn't just dissolve in water; Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. Note that ammonia forms ammonium salts when it reacts with acids. Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. Ammonia is a weak base because its nitrogen atom has an electron pair that readily. Is Ammonia An Acid Base Or Salt.
From slideplayer.com
Identifying acids, bases and salts from their molecular formulas ppt Is Ammonia An Acid Base Or Salt Nh3 (ammonia) is one of. This is a simple example of ammonia reacting with an acid to make a salt. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the. Is Ammonia An Acid Base Or Salt.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089759 Is Ammonia An Acid Base Or Salt Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. This is a simple example of ammonia reacting with an acid to make a salt. Nh3 (ammonia) is one of. Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. Note that ammonia forms ammonium salts when it reacts with acids. Ammonia. Is Ammonia An Acid Base Or Salt.
From www.youtube.com
Ammonium Salts and Solutions Acids, Bases & Alkali's Chemistry Is Ammonia An Acid Base Or Salt Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts. Is Ammonia An Acid Base Or Salt.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Is Ammonia An Acid Base Or Salt Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. For instance, ammonia reacts with hydrochloric acid to make ammonium. Note that ammonia forms ammonium salts when it reacts with acids. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes. Is Ammonia An Acid Base Or Salt.
From www.slideserve.com
PPT Chapter 19 Acids, Bases, and Salts PowerPoint Presentation, free Is Ammonia An Acid Base Or Salt Ammonia doesn't just dissolve in water; Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. In short, nh3, ammonia, it is considered a base but it is a weak base. This is a simple example of ammonia reacting with an acid to make a salt. Although nh3. Is Ammonia An Acid Base Or Salt.
From techiescientist.com
Is NH3 (Ammonia) an Acid or a Base? Techiescientist Is Ammonia An Acid Base Or Salt For instance, ammonia reacts with hydrochloric acid to make ammonium. Note that ammonia forms ammonium salts when it reacts with acids. Ammonia doesn't just dissolve in water; Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. Ammonia is a weak base because its nitrogen atom has an electron pair. Is Ammonia An Acid Base Or Salt.
From www.slideserve.com
PPT Acids And Bases PowerPoint Presentation, free download ID3761395 Is Ammonia An Acid Base Or Salt Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. Nh3 (ammonia) is one of. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. Ammonia doesn't just dissolve in water; As explained earlier, nh3 is a weak base and reacts. Is Ammonia An Acid Base Or Salt.
From www.scienceabc.com
Is Ammonia An Acid Or Base? » ScienceABC Is Ammonia An Acid Base Or Salt Note that ammonia forms ammonium salts when it reacts with acids. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. In short, nh3, ammonia, it is considered a base but it is a weak base. Ammonia is a weak base because its nitrogen atom has an electron. Is Ammonia An Acid Base Or Salt.
From jonathanareschapman.blogspot.com
Ammonia Acid or Base JonathanaresChapman Is Ammonia An Acid Base Or Salt Note that ammonia forms ammonium salts when it reacts with acids. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. This is a simple example of ammonia reacting. Is Ammonia An Acid Base Or Salt.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Is Ammonia An Acid Base Or Salt Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. Nh3 (ammonia) is one of. For instance, ammonia reacts with hydrochloric acid to make ammonium. This is a. Is Ammonia An Acid Base Or Salt.
From sciencenotes.org
Facts About Acids and Bases Is Ammonia An Acid Base Or Salt Nh3 (ammonia) is one of. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. As explained earlier, nh3 is a weak base and reacts with acids to form salts. This is a simple example of ammonia reacting with an. Is Ammonia An Acid Base Or Salt.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses Is Ammonia An Acid Base Or Salt Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. For instance, ammonia reacts with hydrochloric acid to make ammonium. As explained earlier, nh3 is a weak base and reacts with acids to form salts. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. Nh3 (ammonia) is one of.. Is Ammonia An Acid Base Or Salt.
From www.differencebetween.com
Difference Between Acidic Salt and Basic Salt Compare the Difference Is Ammonia An Acid Base Or Salt In short, nh3, ammonia, it is considered a base but it is a weak base. Nh3 (ammonia) is one of. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it. Is Ammonia An Acid Base Or Salt.
From slideplayer.com
Learning Objectives Acids and Alkalis ppt download Is Ammonia An Acid Base Or Salt Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. In short, nh3, ammonia, it is considered a base but it is a weak base. For instance, ammonia reacts with hydrochloric acid to make ammonium. Note that ammonia forms ammonium salts when it reacts with acids. Acid +. Is Ammonia An Acid Base Or Salt.
From draweasy8.blogspot.com
(nh4)2so4 Acid Base Or Salt Draw Easy Is Ammonia An Acid Base Or Salt Note that ammonia forms ammonium salts when it reacts with acids. This is a simple example of ammonia reacting with an acid to make a salt. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. Nh3 (ammonia) is one of. Acid +. Is Ammonia An Acid Base Or Salt.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Is Ammonia An Acid Base Or Salt This is a simple example of ammonia reacting with an acid to make a salt. Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. In short, nh3, ammonia, it is considered a base but it is a weak base. Nh3 (ammonia) is one of. As explained earlier, nh3 is a weak base and reacts with acids to form salts. Ammonia is. Is Ammonia An Acid Base Or Salt.
From byjus.com
nh4cl is acid but any NH2 is base in liquid ammonia Is Ammonia An Acid Base Or Salt Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. As explained earlier, nh3 is a weak base and reacts with acids to form salts. For instance, ammonia reacts with hydrochloric acid to make ammonium. Nh3 (ammonia) is one of. Although nh3 is a weak base, it also. Is Ammonia An Acid Base Or Salt.
From www.slideserve.com
PPT IX. Acids, Bases and Salts PowerPoint Presentation, free download Is Ammonia An Acid Base Or Salt Note that ammonia forms ammonium salts when it reacts with acids. For instance, ammonia reacts with hydrochloric acid to make ammonium. Ammonia doesn't just dissolve in water; As explained earlier, nh3 is a weak base and reacts with acids to form salts. Nh3 (ammonia) is one of. Ammonia is a weak base because its nitrogen atom has an electron pair. Is Ammonia An Acid Base Or Salt.
From slideplayer.com
Chapter 20 Notes, part I Acids and Bases. ppt download Is Ammonia An Acid Base Or Salt Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. As explained earlier, nh3 is a weak base and reacts with acids to form salts. Note that ammonia forms ammonium salts when it reacts with acids. Nh3 (ammonia) is one of. Ammonia is a weak base because its nitrogen atom has. Is Ammonia An Acid Base Or Salt.
From www.masterorganicchemistry.com
How To Use a pKa Table Is Ammonia An Acid Base Or Salt As explained earlier, nh3 is a weak base and reacts with acids to form salts. Nh3 (ammonia) is one of. Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. For instance, ammonia reacts with hydrochloric acid to make ammonium. Ammonia doesn't just dissolve in water; Ammonia is. Is Ammonia An Acid Base Or Salt.
From sciencenotes.org
The pH Scale of Common Chemicals Is Ammonia An Acid Base Or Salt Note that ammonia forms ammonium salts when it reacts with acids. Nh3 (ammonia) is one of. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. As explained. Is Ammonia An Acid Base Or Salt.
From draweasy8.blogspot.com
(nh4)2so4 Acid Base Or Salt Draw Easy Is Ammonia An Acid Base Or Salt As explained earlier, nh3 is a weak base and reacts with acids to form salts. In short, nh3, ammonia, it is considered a base but it is a weak base. Acid + base gives salt and water is only possible when acid and base both can dissociate into two ions. Ammonia doesn't just dissolve in water; Let's take an example. Is Ammonia An Acid Base Or Salt.
From www.youtube.com
Acidbase properties of salts Acids and bases AP Chemistry Khan Is Ammonia An Acid Base Or Salt As explained earlier, nh3 is a weak base and reacts with acids to form salts. Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which makes it weak. Although nh3 is a weak base, it also acts as a weak acid under certain. Is Ammonia An Acid Base Or Salt.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Is Ammonia An Acid Base Or Salt As explained earlier, nh3 is a weak base and reacts with acids to form salts. Nh3 (ammonia) is one of. Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. For instance, ammonia reacts with hydrochloric acid to make ammonium. Ammonia doesn't just dissolve in water; Note that ammonia forms ammonium salts when it reacts with acids. In short, nh3, ammonia, it. Is Ammonia An Acid Base Or Salt.
From www.youtube.com
Is NH3 (Ammonia) an Acid, Base, or Neutral YouTube Is Ammonia An Acid Base Or Salt For instance, ammonia reacts with hydrochloric acid to make ammonium. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. Note that ammonia forms ammonium salts when it reacts with acids. As explained earlier, nh3 is a weak base and reacts with acids to form salts. Ammonia is a weak base. Is Ammonia An Acid Base Or Salt.
From www.youtube.com
Classifying salt solution as acidic, basic, or neutral YouTube Is Ammonia An Acid Base Or Salt As explained earlier, nh3 is a weak base and reacts with acids to form salts. For instance, ammonia reacts with hydrochloric acid to make ammonium. Let's take an example $\ce{hcl}$ and $\ce{naoh}$ gives. This is a simple example of ammonia reacting with an acid to make a salt. Acid + base gives salt and water is only possible when acid. Is Ammonia An Acid Base Or Salt.
From www.numerade.com
SOLVEDAmines are organic bases that react with water to produce Is Ammonia An Acid Base Or Salt Acidic salt (strong acid/weak base) these are acidic since the cation is the conjugate acid of a weak base, and the weaker. Although nh3 is a weak base, it also acts as a weak acid under certain conditions and reacts with. For instance, ammonia reacts with hydrochloric acid to make ammonium. This is a simple example of ammonia reacting with. Is Ammonia An Acid Base Or Salt.
From topblogtenz.com
Is NH4NO3 an acid or base or salt? Nature of Ammonium nitrate Is Ammonia An Acid Base Or Salt This is a simple example of ammonia reacting with an acid to make a salt. In short, nh3, ammonia, it is considered a base but it is a weak base. Note that ammonia forms ammonium salts when it reacts with acids. Ammonia is a weak base because its nitrogen atom has an electron pair that readily accepts a proton, which. Is Ammonia An Acid Base Or Salt.