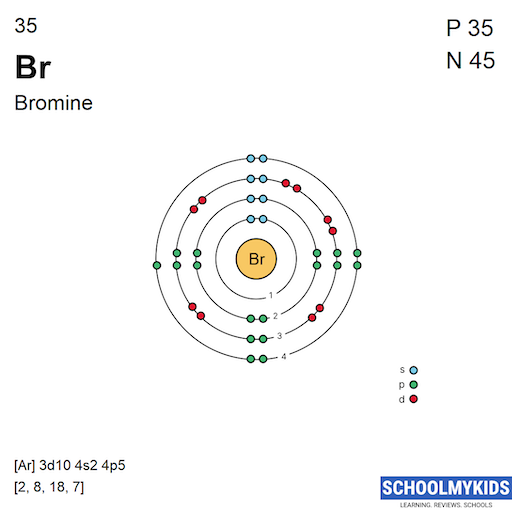
Electron Affinity Chlorine And Bromine . chlorine does have a higher electron affinity than bromine. In each case, a halogen higher in the group can oxidise the ions of one lower down. chlorine, bromine and iodine. the halogens are located on the left of the noble gases on the periodic table. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. it is an extremely reactive element and a strong oxidising agent: As you know, both chlorine and bromine are located in. So, why does that happen? 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or.
from www.schoolmykids.com
chlorine does have a higher electron affinity than bromine. the halogens are located on the left of the noble gases on the periodic table. So, why does that happen? In each case, a halogen higher in the group can oxidise the ions of one lower down. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. As you know, both chlorine and bromine are located in. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. it is an extremely reactive element and a strong oxidising agent: chlorine, bromine and iodine.
Compare Bromine vs Iodine Periodic Table Element Comparison Compare
Electron Affinity Chlorine And Bromine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. So, why does that happen? it is an extremely reactive element and a strong oxidising agent: As you know, both chlorine and bromine are located in. chlorine, bromine and iodine. chlorine does have a higher electron affinity than bromine. In each case, a halogen higher in the group can oxidise the ions of one lower down. the halogens are located on the left of the noble gases on the periodic table. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when.
From www.vedantu.com
Going from fluorine, chlorine, bromine to iodine the electronegativity Electron Affinity Chlorine And Bromine So, why does that happen? In each case, a halogen higher in the group can oxidise the ions of one lower down. the halogens are located on the left of the noble gases on the periodic table. it is an extremely reactive element and a strong oxidising agent: chlorine does have a higher electron affinity than bromine.. Electron Affinity Chlorine And Bromine.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Electron Affinity Chlorine And Bromine 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. the halogens are located on the left of the noble gases on the periodic table. In each case, a halogen higher in the group can oxidise the ions of one lower down. it is. Electron Affinity Chlorine And Bromine.
From www.transtutors.com
(Solved) Exp 9. Lab Assignment Part I Periodic Trends Atomic Radius Electron Affinity Chlorine And Bromine chlorine does have a higher electron affinity than bromine. it is an extremely reactive element and a strong oxidising agent: In each case, a halogen higher in the group can oxidise the ions of one lower down. So, why does that happen? chlorine, bromine and iodine. As you know, both chlorine and bromine are located in. . Electron Affinity Chlorine And Bromine.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Electron Affinity Chlorine And Bromine In each case, a halogen higher in the group can oxidise the ions of one lower down. chlorine does have a higher electron affinity than bromine. chlorine, bromine and iodine. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. As you know, both chlorine and bromine. Electron Affinity Chlorine And Bromine.
From www.pearson.com
The periodic table with electron affinity values is shown below Electron Affinity Chlorine And Bromine chlorine, bromine and iodine. In each case, a halogen higher in the group can oxidise the ions of one lower down. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. it is an extremely reactive element and a strong oxidising agent: the. Electron Affinity Chlorine And Bromine.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Electron Affinity Chlorine And Bromine chlorine does have a higher electron affinity than bromine. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. it is an extremely. Electron Affinity Chlorine And Bromine.
From dxogpncsm.blob.core.windows.net
Bromine Element Protons Neutrons And Electrons at Kelly Moya blog Electron Affinity Chlorine And Bromine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. So, why does that happen? As you know, both chlorine and bromine are located in.. Electron Affinity Chlorine And Bromine.
From mavink.com
Periodic Table With Electron Affinity Electron Affinity Chlorine And Bromine In each case, a halogen higher in the group can oxidise the ions of one lower down. So, why does that happen? it is an extremely reactive element and a strong oxidising agent: the halogens are located on the left of the noble gases on the periodic table. As you know, both chlorine and bromine are located in.. Electron Affinity Chlorine And Bromine.
From festivalegiptoenbarcelona.com
Símbolos y estructuras de Lewis CHEM 1305 Introductory Chemistry El Electron Affinity Chlorine And Bromine So, why does that happen? In each case, a halogen higher in the group can oxidise the ions of one lower down. chlorine does have a higher electron affinity than bromine. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. it is an. Electron Affinity Chlorine And Bromine.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Electron Affinity Chlorine And Bromine As you know, both chlorine and bromine are located in. it is an extremely reactive element and a strong oxidising agent: chlorine does have a higher electron affinity than bromine. So, why does that happen? 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom. Electron Affinity Chlorine And Bromine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Electron Affinity Chlorine And Bromine chlorine does have a higher electron affinity than bromine. In each case, a halogen higher in the group can oxidise the ions of one lower down. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. As you know, both chlorine and bromine are located. Electron Affinity Chlorine And Bromine.
From www.chegg.com
Solved Electron Affinity Chlorine Flourine Bromine Electron Electron Affinity Chlorine And Bromine chlorine does have a higher electron affinity than bromine. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. So, why does that happen? 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or.. Electron Affinity Chlorine And Bromine.
From www.pinterest.com
Electron Affinity Electron affinity, Periodic table, Chemistry for kids Electron Affinity Chlorine And Bromine In each case, a halogen higher in the group can oxidise the ions of one lower down. it is an extremely reactive element and a strong oxidising agent: So, why does that happen? chlorine does have a higher electron affinity than bromine. the halogens are located on the left of the noble gases on the periodic table.. Electron Affinity Chlorine And Bromine.
From www.doubtnut.com
The electron affinity of bromine atom is equal to the..........of Electron Affinity Chlorine And Bromine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. chlorine does have a higher electron affinity than bromine. it is an extremely reactive element and a strong oxidising agent: the halogens are located on the left of the noble gases on the periodic table. As. Electron Affinity Chlorine And Bromine.
From material-properties.org
Bromo Protones Neutrones Electrones Configuración electrónica Electron Affinity Chlorine And Bromine the halogens are located on the left of the noble gases on the periodic table. As you know, both chlorine and bromine are located in. So, why does that happen? electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. it is an extremely reactive element and. Electron Affinity Chlorine And Bromine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Electron Affinity Chlorine And Bromine chlorine does have a higher electron affinity than bromine. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. chlorine, bromine and iodine. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when.. Electron Affinity Chlorine And Bromine.
From www.numerade.com
SOLVEDUse electron configurations to explain why (a) sulfur has a Electron Affinity Chlorine And Bromine In each case, a halogen higher in the group can oxidise the ions of one lower down. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. As you know, both chlorine and bromine are located in. chlorine, bromine and iodine. So, why does that happen? it. Electron Affinity Chlorine And Bromine.
From www.youtube.com
Electron Affinity Trend, Basic Introduction, Chemistry YouTube Electron Affinity Chlorine And Bromine So, why does that happen? electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. In each case, a halogen higher in the group can oxidise the ions of one lower down. chlorine, bromine and iodine. chlorine does have a higher electron affinity than bromine. As you. Electron Affinity Chlorine And Bromine.
From www.meritnation.com
Does chlorine have higher electron affinity value than fluorine Electron Affinity Chlorine And Bromine chlorine, bromine and iodine. chlorine does have a higher electron affinity than bromine. As you know, both chlorine and bromine are located in. So, why does that happen? 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. In each case, a halogen higher. Electron Affinity Chlorine And Bromine.
From www.numerade.com
SOLVED While the electron affinity of bromine is a negative quantity Electron Affinity Chlorine And Bromine In each case, a halogen higher in the group can oxidise the ions of one lower down. So, why does that happen? 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in energy (in kj/mole) of a. Electron Affinity Chlorine And Bromine.
From brainly.com
Rank the following elements by electron affinity, from most positive to Electron Affinity Chlorine And Bromine In each case, a halogen higher in the group can oxidise the ions of one lower down. As you know, both chlorine and bromine are located in. it is an extremely reactive element and a strong oxidising agent: 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a. Electron Affinity Chlorine And Bromine.
From ar.inspiredpencil.com
Electron Affinity Equation Electron Affinity Chlorine And Bromine 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. In each case, a halogen higher in the group can oxidise the ions of one lower down. So, why does that happen? chlorine, bromine and iodine. it is an extremely reactive element and a. Electron Affinity Chlorine And Bromine.
From www.toppr.com
The electron affinity of bromine atom is equal to the Electron Affinity Chlorine And Bromine the halogens are located on the left of the noble gases on the periodic table. As you know, both chlorine and bromine are located in. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. So, why does that happen? it is an extremely reactive element and. Electron Affinity Chlorine And Bromine.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare Electron Affinity Chlorine And Bromine the halogens are located on the left of the noble gases on the periodic table. So, why does that happen? chlorine does have a higher electron affinity than bromine. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. chlorine, bromine and iodine.. Electron Affinity Chlorine And Bromine.
From dxoqxvxev.blob.core.windows.net
How Many Valence Electrons Does Bromine Want at Jan Myers blog Electron Affinity Chlorine And Bromine the halogens are located on the left of the noble gases on the periodic table. So, why does that happen? 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in energy (in kj/mole) of a neutral. Electron Affinity Chlorine And Bromine.
From slideplayer.com
Choice B Br2(l) Br2(g) 2 Br(g) 2 Br+(g) + 2e ppt download Electron Affinity Chlorine And Bromine As you know, both chlorine and bromine are located in. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. the halogens are located on the left of the noble gases on the periodic table. 125 rows the electron affinity is defined as the amount of energy. Electron Affinity Chlorine And Bromine.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Electron Affinity Chlorine And Bromine In each case, a halogen higher in the group can oxidise the ions of one lower down. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. it is an extremely reactive element and a strong oxidising agent: 125 rows the electron affinity is defined as the. Electron Affinity Chlorine And Bromine.
From www.angelo.edu
The Parts of the Periodic Table Electron Affinity Chlorine And Bromine chlorine does have a higher electron affinity than bromine. the halogens are located on the left of the noble gases on the periodic table. So, why does that happen? As you know, both chlorine and bromine are located in. it is an extremely reactive element and a strong oxidising agent: chlorine, bromine and iodine. 125. Electron Affinity Chlorine And Bromine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Electron Affinity Chlorine And Bromine chlorine, bromine and iodine. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. So, why does that happen? chlorine does have a. Electron Affinity Chlorine And Bromine.
From www.youtube.com
Halogens second electron Affinity is zero Why?Fluorine chlorine bromine Electron Affinity Chlorine And Bromine chlorine does have a higher electron affinity than bromine. As you know, both chlorine and bromine are located in. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. it is an extremely reactive element and a strong oxidising agent: the halogens are located on the. Electron Affinity Chlorine And Bromine.
From ppt-online.org
The Periodic Table презентация онлайн Electron Affinity Chlorine And Bromine chlorine, bromine and iodine. So, why does that happen? In each case, a halogen higher in the group can oxidise the ions of one lower down. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 125 rows the electron affinity is defined as the amount of. Electron Affinity Chlorine And Bromine.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Electron Affinity Chlorine And Bromine In each case, a halogen higher in the group can oxidise the ions of one lower down. it is an extremely reactive element and a strong oxidising agent: chlorine, bromine and iodine. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. electron. Electron Affinity Chlorine And Bromine.
From www.pinterest.com
Electron Affinity Trend and Definition Electron affinity, Electrons Electron Affinity Chlorine And Bromine As you know, both chlorine and bromine are located in. chlorine, bromine and iodine. So, why does that happen? the halogens are located on the left of the noble gases on the periodic table. chlorine does have a higher electron affinity than bromine. In each case, a halogen higher in the group can oxidise the ions of. Electron Affinity Chlorine And Bromine.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Electron Affinity Chlorine And Bromine electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. it is an extremely reactive element and a strong oxidising agent: chlorine, bromine and iodine. chlorine does have a higher electron affinity than bromine. 125 rows the electron affinity is defined as the amount of. Electron Affinity Chlorine And Bromine.
From www.doubtnut.com
The electron affinity of bromine (g) is 3.9 eV. How much energy in kJ Electron Affinity Chlorine And Bromine chlorine, bromine and iodine. As you know, both chlorine and bromine are located in. 125 rows the electron affinity is defined as the amount of energy released when an electron is added to a neutral atom or. In each case, a halogen higher in the group can oxidise the ions of one lower down. it is an. Electron Affinity Chlorine And Bromine.