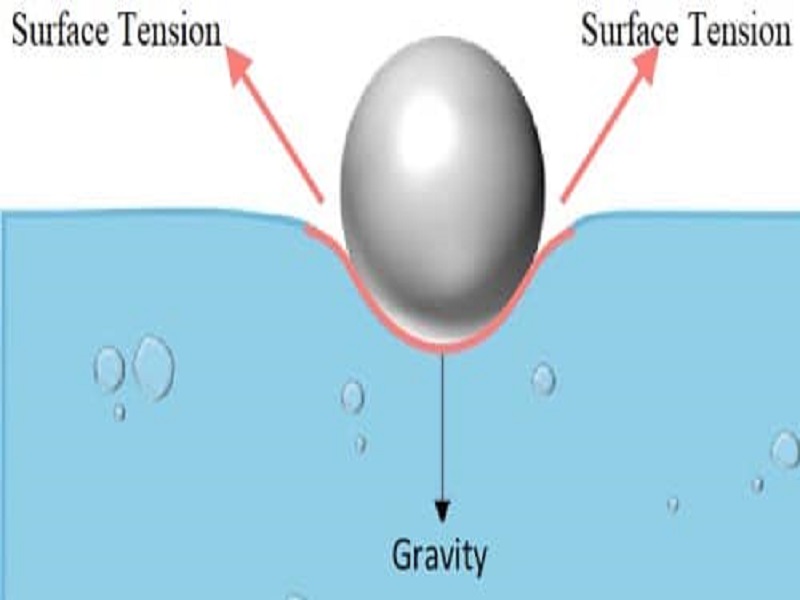
How Does Water Have High Surface Tension . among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is a manifestation. besides mercury, water has the highest surface tension for all liquids. next to mercury, water has the highest surface tension of all commonly occurring liquids. As a result of this high surface. The cohesive forces between liquid molecules. In comparison, organic liquids, such as benzene and alcohols ,. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Water's high surface tension is due to the.
from studiousguy.com
water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). besides mercury, water has the highest surface tension for all liquids. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. next to mercury, water has the highest surface tension of all commonly occurring liquids. The cohesive forces between liquid molecules. In comparison, organic liquids, such as benzene and alcohols ,. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Water's high surface tension is due to the. As a result of this high surface. Surface tension is a manifestation.
10 Surface Tension Examples in Daily Life StudiousGuy
How Does Water Have High Surface Tension Surface tension is a manifestation. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). next to mercury, water has the highest surface tension of all commonly occurring liquids. besides mercury, water has the highest surface tension for all liquids. The cohesive forces between liquid molecules. Water's high surface tension is due to the. In comparison, organic liquids, such as benzene and alcohols ,. Surface tension is a manifestation. As a result of this high surface. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How Does Water Have High Surface Tension Surface tension is a manifestation. As a result of this high surface. besides mercury, water has the highest surface tension for all liquids. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. among common liquids, water exhibits a distinctly high surface tension due to. How Does Water Have High Surface Tension.
From studyschoollifebelts.z21.web.core.windows.net
How To Explain Surface Tension How Does Water Have High Surface Tension As a result of this high surface. Surface tension is a manifestation. The cohesive forces between liquid molecules. besides mercury, water has the highest surface tension for all liquids. In comparison, organic liquids, such as benzene and alcohols ,. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water's high surface. How Does Water Have High Surface Tension.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Water Have High Surface Tension The cohesive forces between liquid molecules. besides mercury, water has the highest surface tension for all liquids. Water's high surface tension is due to the. Surface tension is a manifestation. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). among common liquids, water exhibits a distinctly high surface tension due. How Does Water Have High Surface Tension.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts How Does Water Have High Surface Tension surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Surface tension is a manifestation. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Water's high surface tension is due to the. next to mercury, water. How Does Water Have High Surface Tension.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation ID565121 How Does Water Have High Surface Tension The cohesive forces between liquid molecules. In comparison, organic liquids, such as benzene and alcohols ,. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). . How Does Water Have High Surface Tension.
From circuitwiringtray.z13.web.core.windows.net
Surface Tension Of Water Diagram How Does Water Have High Surface Tension Water's high surface tension is due to the. next to mercury, water has the highest surface tension of all commonly occurring liquids. As a result of this high surface. The cohesive forces between liquid molecules. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. . How Does Water Have High Surface Tension.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download How Does Water Have High Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols ,. besides mercury, water has the highest surface tension for all liquids. As a result of this high surface. Surface tension is a manifestation. The cohesive forces between liquid molecules. next to. How Does Water Have High Surface Tension.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension How Does Water Have High Surface Tension Water's high surface tension is due to the. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols ,. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Surface tension. How Does Water Have High Surface Tension.
From forum.grasscity.com
Is this all i need to start making organic soil ? Page 2 Grasscity How Does Water Have High Surface Tension As a result of this high surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. In comparison, organic liquids, such as benzene and alcohols ,. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between liquid molecules. Water's high surface. How Does Water Have High Surface Tension.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts How Does Water Have High Surface Tension As a result of this high surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water's high surface tension is due to the. Surface tension is a manifestation. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. surface tension of. How Does Water Have High Surface Tension.
From funsizephysics.com
What is Surface Tension? FunsizePhysics How Does Water Have High Surface Tension The cohesive forces between liquid molecules. next to mercury, water has the highest surface tension of all commonly occurring liquids. As a result of this high surface. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is a manifestation. water has a surface tension of 0.07275. How Does Water Have High Surface Tension.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? How Does Water Have High Surface Tension As a result of this high surface. The cohesive forces between liquid molecules. Surface tension is a manifestation. In comparison, organic liquids, such as benzene and alcohols ,. next to mercury, water has the highest surface tension of all commonly occurring liquids. Water's high surface tension is due to the. besides mercury, water has the highest surface tension. How Does Water Have High Surface Tension.
From www.youtube.com
Surface tension of water YouTube How Does Water Have High Surface Tension next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Water's high surface. How Does Water Have High Surface Tension.
From www.youtube.com
Surface Tension of Water Explained YouTube How Does Water Have High Surface Tension surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. The cohesive forces between liquid molecules. besides mercury, water has the highest surface tension for all liquids. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules.. How Does Water Have High Surface Tension.
From www.appgecet.co.in
Why Does Water Have High Surface Tension But Low Viscosity? AP PGECET How Does Water Have High Surface Tension Surface tension is a manifestation. The cohesive forces between liquid molecules. next to mercury, water has the highest surface tension of all commonly occurring liquids. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). As a result of this high surface. surface tension of water can cause things to float. How Does Water Have High Surface Tension.
From www.youtube.com
Surface Tension YouTube How Does Water Have High Surface Tension The cohesive forces between liquid molecules. Surface tension is a manifestation. besides mercury, water has the highest surface tension for all liquids. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. among common liquids, water exhibits a distinctly high surface tension due to strong. How Does Water Have High Surface Tension.
From www.whatdowedoallday.com
Surface Tension Experiment Water Drop Races How Does Water Have High Surface Tension Water's high surface tension is due to the. As a result of this high surface. besides mercury, water has the highest surface tension for all liquids. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. surface tension of water can cause things to float which are denser than. How Does Water Have High Surface Tension.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid How Does Water Have High Surface Tension As a result of this high surface. besides mercury, water has the highest surface tension for all liquids. In comparison, organic liquids, such as benzene and alcohols ,. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is a manifestation. next to mercury, water has the. How Does Water Have High Surface Tension.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download How Does Water Have High Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is a manifestation. besides mercury, water has the highest surface tension for all liquids. next to mercury, water has the highest surface tension of all commonly occurring liquids. In comparison, organic liquids, such as benzene and alcohols. How Does Water Have High Surface Tension.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids How Does Water Have High Surface Tension Water's high surface tension is due to the. As a result of this high surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols ,. The cohesive forces between liquid molecules. next to mercury, water has the highest surface tension of all. How Does Water Have High Surface Tension.
From sciencewithkids.com
Water Surface Tension Experiment Science with How Does Water Have High Surface Tension As a result of this high surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. The cohesive forces between liquid molecules. Water's high surface tension is due to the. Surface tension is a manifestation. In comparison, organic liquids, such as benzene and alcohols ,. besides mercury, water has the highest surface tension. How Does Water Have High Surface Tension.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog How Does Water Have High Surface Tension surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water's high surface tension is due to the. besides mercury, water has the highest surface tension for all liquids.. How Does Water Have High Surface Tension.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does Water Have High Surface Tension The cohesive forces between liquid molecules. As a result of this high surface. Surface tension is a manifestation. besides mercury, water has the highest surface tension for all liquids. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. In comparison, organic liquids, such as benzene and alcohols ,. Water's. How Does Water Have High Surface Tension.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog How Does Water Have High Surface Tension next to mercury, water has the highest surface tension of all commonly occurring liquids. Water's high surface tension is due to the. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. besides mercury, water has the highest surface tension for all liquids. As a result of this high. How Does Water Have High Surface Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Does Water Have High Surface Tension besides mercury, water has the highest surface tension for all liquids. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Water's high surface tension is due to the. water. How Does Water Have High Surface Tension.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with How Does Water Have High Surface Tension In comparison, organic liquids, such as benzene and alcohols ,. besides mercury, water has the highest surface tension for all liquids. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Surface tension is a manifestation. next to mercury, water has the highest surface tension of all commonly occurring liquids. Water's. How Does Water Have High Surface Tension.
From ar.inspiredpencil.com
Surface Tension Water How Does Water Have High Surface Tension In comparison, organic liquids, such as benzene and alcohols ,. next to mercury, water has the highest surface tension of all commonly occurring liquids. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68. How Does Water Have High Surface Tension.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog How Does Water Have High Surface Tension As a result of this high surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. The cohesive forces between liquid molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. water has a surface tension of 0.07275 joule per square metre at. How Does Water Have High Surface Tension.
From www.appgecet.co.in
Why Does Water Have High Surface Tension But Low Viscosity? AP PGECET How Does Water Have High Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. As a result of this high surface. besides mercury, water has the highest surface tension for all. How Does Water Have High Surface Tension.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does Water Have High Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. besides mercury, water has the highest surface tension for all liquids. Surface tension is a manifestation. As a result of this high surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. In comparison,. How Does Water Have High Surface Tension.
From hxeafvitb.blob.core.windows.net
Why Water Have High Surface Tension at Nancy Aguirre blog How Does Water Have High Surface Tension In comparison, organic liquids, such as benzene and alcohols ,. besides mercury, water has the highest surface tension for all liquids. Water's high surface tension is due to the. Surface tension is a manifestation. The cohesive forces between liquid molecules. among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules.. How Does Water Have High Surface Tension.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation How Does Water Have High Surface Tension Water's high surface tension is due to the. The cohesive forces between liquid molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). besides mercury, water has the highest surface tension for all liquids. In comparison, organic liquids, such as benzene and alcohols ,. As a result of this high surface.. How Does Water Have High Surface Tension.
From www.moleaer.com
How nanobubbles improve water infiltration by reducing surface tension How Does Water Have High Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. besides mercury, water has the highest surface tension for all liquids. In comparison, organic liquids, such as benzene and alcohols ,. next to mercury, water has the highest surface tension of all commonly occurring liquids. Surface tension is a. How Does Water Have High Surface Tension.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii How Does Water Have High Surface Tension The cohesive forces between liquid molecules. surface tension of water can cause things to float which are denser than water, allowing organisms to literally walk on water (figure. Surface tension is a manifestation. next to mercury, water has the highest surface tension of all commonly occurring liquids. among common liquids, water exhibits a distinctly high surface tension. How Does Water Have High Surface Tension.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments How Does Water Have High Surface Tension among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. The cohesive forces between liquid molecules. As a result of this high surface. next to mercury, water has the highest surface tension of all commonly occurring liquids. Water's high surface tension is due to the. surface tension of water. How Does Water Have High Surface Tension.