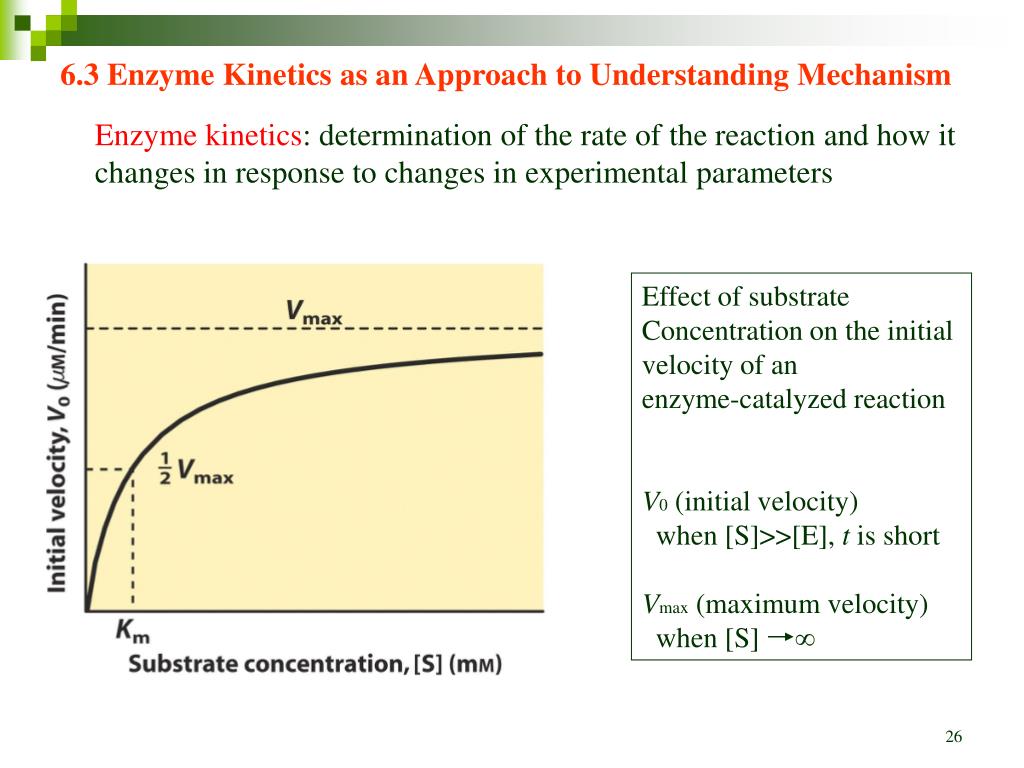
Enzyme Kinetics Differential Equations . Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Another important advancement which, in combination with. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzyme kinetics is a key aspect of biochemistry that examines the rates of.
from www.slideserve.com
The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Another important advancement which, in combination with. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate.
PPT Chapter 6 Enzymes PowerPoint Presentation, free download ID5143485
Enzyme Kinetics Differential Equations The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Another important advancement which, in combination with.
From www.slideserve.com
PPT 10 Equations in Biology MichaelisMenten PowerPoint Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. The law of mass action applied to this system leads. Enzyme Kinetics Differential Equations.
From www.scribd.com
Equations in Enzyme PDF Enzyme Gibbs Free Energy Enzyme Kinetics Differential Equations Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. Another important advancement which, in combination with. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the. Enzyme Kinetics Differential Equations.
From www.sqadia.com
Enzyme I Introduction Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. Another important advancement which, in combination with. The law of mass action applied. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID2045841 Enzyme Kinetics Differential Equations The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. For example,. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Lecture 4 The of EnzymeCatalyzed Reactions PowerPoint Enzyme Kinetics Differential Equations Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid.. Enzyme Kinetics Differential Equations.
From www.chegg.com
Solved Match the equation to its purpose a. Describes Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. Another important advancement which, in combination with. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. The law of. Enzyme Kinetics Differential Equations.
From www.imagwiki.nibib.nih.gov
A Guide for MichaelisMenten Enzyme Models (MICMEN Enzyme Kinetics Differential Equations The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Another important advancement which, in combination with. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzyme. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT 10 Equations in Biology MichaelisMenten PowerPoint Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate.. Enzyme Kinetics Differential Equations.
From www.semanticscholar.org
Table 2 from Application of Differential Equations in Enzyme Enzyme Kinetics Differential Equations Another important advancement which, in combination with. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. The law of. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 Enzyme Kinetics Differential Equations Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Another important advancement which, in combination with. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: For example, the enzyme acetylcholinesterase. Enzyme Kinetics Differential Equations.
From chem.libretexts.org
10.2 The Equations of Enzyme Chemistry LibreTexts Enzyme Kinetics Differential Equations Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. Another important advancement which, in combination with. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. The law of. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate.. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID2045841 Enzyme Kinetics Differential Equations Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Another important advancement which, in combination with. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: For example, the enzyme acetylcholinesterase. Enzyme Kinetics Differential Equations.
From www.scribd.com
3 Enzyme Enzyme Chemical Reactions Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Another important advancement which, in. Enzyme Kinetics Differential Equations.
From www.semanticscholar.org
Figure 1 from Mathematical treatment of enzyme using Enzyme Kinetics Differential Equations Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Enzyme kinetics is a key aspect of biochemistry. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID9573440 Enzyme Kinetics Differential Equations Enzyme kinetics is a key aspect of biochemistry that examines the rates of. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Another important advancement which, in combination with. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Chapter 12 Enzyme Inhibition, and Control PowerPoint Enzyme Kinetics Differential Equations The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Another important advancement which, in combination with. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant,. Enzyme Kinetics Differential Equations.
From chem.libretexts.org
10.2 The Equations of Enzyme Chemistry LibreTexts Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Another important advancement which, in combination with. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzyme. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT 10 Equations in Biology MichaelisMenten PowerPoint Enzyme Kinetics Differential Equations Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. The law of mass action applied to this system leads to the following. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Enzyme Study the rate of enzyme catalyzed reactions Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant,. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Chapter 6 Enzymes PowerPoint Presentation, free download ID5143485 Enzyme Kinetics Differential Equations Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c.. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Numerical Enzyme using DynaFit software PowerPoint Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate.. Enzyme Kinetics Differential Equations.
From www.scribd.com
BIO307 Lecture (Enzyme I) PDF Active Site Enzyme Enzyme Kinetics Differential Equations Enzyme kinetics is a key aspect of biochemistry that examines the rates of. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Another important advancement which, in combination with. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID6630235 Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Another important advancement which, in combination with. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzymes. Enzyme Kinetics Differential Equations.
From jackwestin.com
Control Of Enzyme Activity MCAT Content Enzyme Kinetics Differential Equations Another important advancement which, in combination with. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 Enzyme Kinetics Differential Equations Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. Another important advancement which, in combination with. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Chapter 13 Enzyme PowerPoint Presentation ID306340 Enzyme Kinetics Differential Equations Another important advancement which, in combination with. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Chapter 6.3 Enzyme PowerPoint Presentation, free Enzyme Kinetics Differential Equations For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Chapter 13 Enzyme PowerPoint Presentation ID306340 Enzyme Kinetics Differential Equations Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. The law of mass action applied to this system leads to the following. Enzyme Kinetics Differential Equations.
From studylib.net
Enzyme Instructor’s Guide Differential Equations Series Table Enzyme Kinetics Differential Equations Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Another important advancement which, in. Enzyme Kinetics Differential Equations.
From www.studocu.com
Enzyme the MichaelisMenten equation Rate Equations k 1 (1 Enzyme Kinetics Differential Equations Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. Another important advancement which, in combination with. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic. Enzyme Kinetics Differential Equations.
From www.slideshare.net
Enzyme Enzyme Kinetics Differential Equations The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Another important advancement which, in combination with. Enzymes. Enzyme Kinetics Differential Equations.
From www.semanticscholar.org
Figure 2 from Analytical solution of coupled second order Enzyme Kinetics Differential Equations The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and. Enzyme Kinetics Differential Equations.
From www.chegg.com
2. Enzyme (MichaelisMenten Equations) The Enzyme Kinetics Differential Equations Another important advancement which, in combination with. Enzymes are highly specific catalysts for biochemical reactions, with each enzyme showing a selectivity for a single reactant, or substrate. Enzyme kinetics is a key aspect of biochemistry that examines the rates of. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine to choline and acetic acid. Ds dt =−k1es+. Enzyme Kinetics Differential Equations.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID196477 Enzyme Kinetics Differential Equations Ds dt =−k1es+ k−1c, de dt =−k1es+ (k−1 + k2)c. The law of mass action applied to this system leads to the following four differential equations that describe the kinetics of the basic enzyme reaction: Enzyme kinetics is a key aspect of biochemistry that examines the rates of. For example, the enzyme acetylcholinesterase catalyzes the decomposition of the neurotransmitter acetylcholine. Enzyme Kinetics Differential Equations.