Laboratory Clia Lookup . on january 16, 2024, cdc retired its clia laboratory search tool. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. enter any combination of fields and select search. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. You can find laboratory demographic information on the. You can use the analyte drop down box to select a. The clia lookup by npi or clia npi. the clia program sets standards for clinical laboratory testing and issues certificates.
from www.slideshare.net
the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. You can find laboratory demographic information on the. on january 16, 2024, cdc retired its clia laboratory search tool. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. The clia lookup by npi or clia npi. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. enter any combination of fields and select search. the clia program sets standards for clinical laboratory testing and issues certificates. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. You can use the analyte drop down box to select a.
Setting Up a CLIA Lab
Laboratory Clia Lookup You can find laboratory demographic information on the. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. on january 16, 2024, cdc retired its clia laboratory search tool. You can use the analyte drop down box to select a. the clia program sets standards for clinical laboratory testing and issues certificates. enter any combination of fields and select search. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. The clia lookup by npi or clia npi. You can find laboratory demographic information on the. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been.
From www.sohu.com
经验分享:实验室认证认可之:CLIA和CAP简介_临床 Laboratory Clia Lookup cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. You can use the analyte drop down box to select a. enter any combination of fields and select search. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. You can find laboratory demographic information. Laboratory Clia Lookup.
From support.pmhelpcenter.com
CLIA Number Laboratory Clia Lookup the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. enter any combination of fields and select search. You can find laboratory demographic information on the. You can use the analyte drop down box to select a. the clia program. Laboratory Clia Lookup.
From knovator.com
What Is Clinical Laboratory Improvement Amendments (CLIA)? Laboratory Clia Lookup the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. You can find laboratory demographic information on the. You can use the analyte drop down box to select a. on january 16, 2024, cdc retired its clia laboratory search tool. The clia lookup by npi or clia npi. the clia program sets standards for clinical. Laboratory Clia Lookup.
From medicine.utah.edu
Laboratory Credentials Dermatology U of U School of Medicine Laboratory Clia Lookup enter any combination of fields and select search. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. on january 16, 2024, cdc retired its clia laboratory search tool. You can find laboratory demographic information on the. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have. Laboratory Clia Lookup.
From genincode.com
cliacertificate Testing For Personalised Health Care and Medicine Laboratory Clia Lookup You can use the analyte drop down box to select a. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. on january 16, 2024, cdc retired its clia laboratory search tool. You can find laboratory demographic information on the. enter any combination of fields and select search. the clia database, updated. Laboratory Clia Lookup.
From www.thermofisher.com
Working with CLIA Lab Regulations LIMS System Laboratory Clia Lookup The clia lookup by npi or clia npi. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. You can find laboratory demographic information on the. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. the clinical laboratory improvement amendments of 1988 (clia) regulations. Laboratory Clia Lookup.
From shop.aad.org
Clinical Laboratory Improvement Amendments (CLIA) for Dermatology AAD Shop Laboratory Clia Lookup the clia program sets standards for clinical laboratory testing and issues certificates. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. on january 16, 2024, cdc retired its clia laboratory search tool. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. enter any combination of. Laboratory Clia Lookup.
From iqlabconsultants.com
Ensuring Compliance In Your Lab CLIA Regulations Demystified Lab Consulting Services Laboratory Clia Lookup enter any combination of fields and select search. You can use the analyte drop down box to select a. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. the clia program sets standards for clinical laboratory testing and issues certificates. on january 16, 2024, cdc retired its clia laboratory search tool.. Laboratory Clia Lookup.
From www.mastertrial.com
FDA Clinical Laboratory Improvement Amendments (CLIA) Mastertrial Laboratory Clia Lookup clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. You can use the analyte drop down box to select a. The clia lookup by npi or clia npi. enter any combination of fields and select search. You can find laboratory demographic information on the. the clia database, updated monthly, lists records of. Laboratory Clia Lookup.
From www.slideshare.net
Setting Up a CLIA Lab Laboratory Clia Lookup the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. The clia lookup by npi or clia npi. You can find laboratory demographic information on the. enter any combination of fields and select search. clinical laboratory improvement amendments. Laboratory Clia Lookup.
From www.qps.com
CLIAcertified Laboratory CLIA Certified Lab QPS Laboratory Clia Lookup the clia program sets standards for clinical laboratory testing and issues certificates. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. enter any combination of fields and select search. You can find laboratory demographic information on the. You can use the analyte drop down box to select a. on. Laboratory Clia Lookup.
From www.researchgate.net
(PDF) CLIA requirements for proficiency testing the basics for laboratory professionals Laboratory Clia Lookup enter any combination of fields and select search. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. on january 16, 2024, cdc retired its clia laboratory search tool. the clinical laboratory improvement amendments of. Laboratory Clia Lookup.
From www.diacarta.com
Clia Lab About Us DiaCarta, Inc. Laboratory Clia Lookup the clia program sets standards for clinical laboratory testing and issues certificates. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. You can find laboratory demographic information on the. You can use the analyte drop down box to select a. enter any combination of fields and select search. on. Laboratory Clia Lookup.
From www.prolisphere.com
What is CLIA Certification and How do I get CLIA certification? Laboratory Clia Lookup cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. You can find laboratory demographic information on the. The clia lookup by npi or clia npi. the clia program sets standards for clinical laboratory testing and issues certificates. You can use the analyte drop down box to select a. the clia. Laboratory Clia Lookup.
From www.slideshare.net
Setting Up a CLIA Lab Laboratory Clia Lookup the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. The clia lookup by npi or clia npi. the clia program sets standards for clinical laboratory testing and issues certificates. clinical laboratory improvement amendments (clia) how to apply. Laboratory Clia Lookup.
From www.qps.com
CLIAcertified Laboratory CLIA Certified Lab QPS Laboratory Clia Lookup the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. The clia lookup by npi or clia npi. You can use the analyte drop down. Laboratory Clia Lookup.
From www.lighthouselabservices.com
What Is a CLIA Lab Director and What Are Their Requirements? Laboratory Clia Lookup clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. on january 16, 2024, cdc retired its clia laboratory search tool. enter any combination of fields and select search. You can use the analyte drop down box to select a. the clia program sets standards for clinical laboratory testing and issues certificates.. Laboratory Clia Lookup.
From hometestbox.com
What Is a CLIACertified Lab? Home Test Box Laboratory Clia Lookup You can use the analyte drop down box to select a. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. The clia lookup by npi or clia npi. You can find laboratory demographic information on the. enter any combination of fields and select search. the clinical laboratory improvement amendments of. Laboratory Clia Lookup.
From 3billion.io
CLIA Certification, is it important? Laboratory Clia Lookup the clia program sets standards for clinical laboratory testing and issues certificates. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. The clia lookup by npi or clia npi. You can find laboratory demographic. Laboratory Clia Lookup.
From 3billion.io
CLIA Certification, is it important? Laboratory Clia Lookup on january 16, 2024, cdc retired its clia laboratory search tool. You can find laboratory demographic information on the. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. the clinical laboratory improvement amendments of 1988. Laboratory Clia Lookup.
From www.youtube.com
2020 04 14 ACIL Clinical Laboratory Improvement Amendments CLIA Certification Process inar Laboratory Clia Lookup cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. enter any combination of fields and select search. The clia lookup by npi or clia npi. on january 16, 2024, cdc retired its clia. Laboratory Clia Lookup.
From www.slideshare.net
Setting Up a CLIA Lab Laboratory Clia Lookup the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. on january 16, 2024, cdc retired its clia laboratory search tool. the clia program sets standards for clinical laboratory testing and issues certificates. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. . Laboratory Clia Lookup.
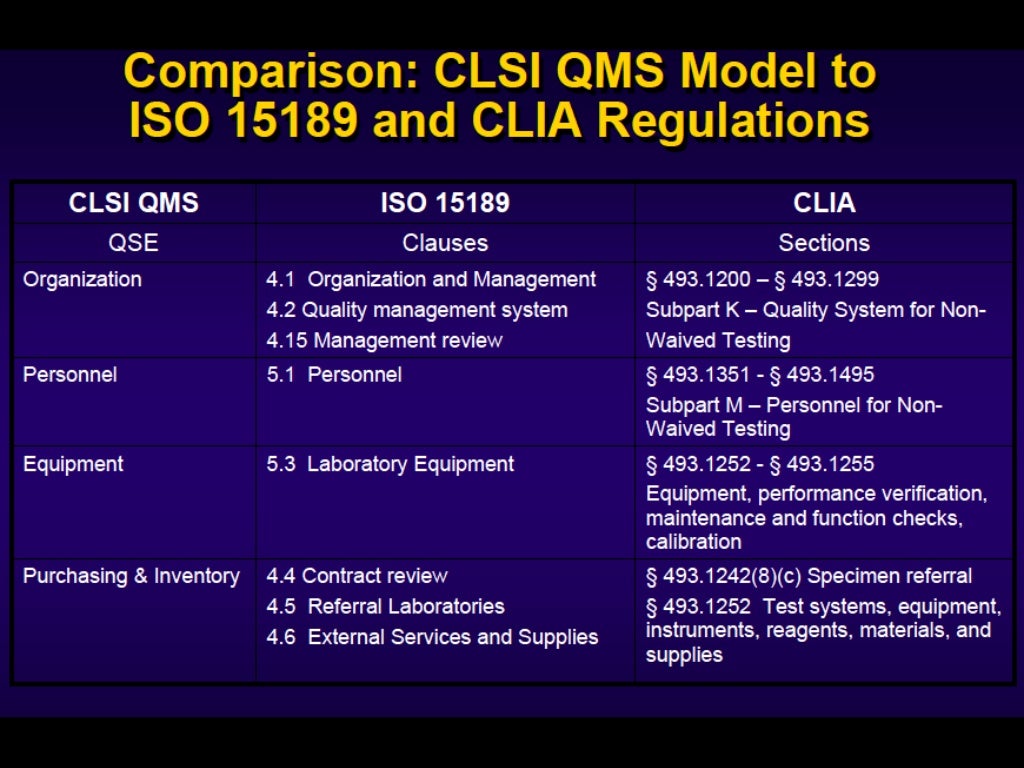
From www.researchgate.net
Overview of Clinical Laboratory Improvement Amendments (CLIA) model,... Download Table Laboratory Clia Lookup on january 16, 2024, cdc retired its clia laboratory search tool. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. The clia lookup by npi or clia npi. the clia program sets standards for clinical laboratory testing and issues certificates. You can use the analyte drop down box to select. Laboratory Clia Lookup.
From www.collaborativetherapeutics.com
Ultimate CLIA Lab Building Checklist Laboratory Clia Lookup clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. on january 16, 2024, cdc retired its clia laboratory search tool. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the clia program sets standards for clinical laboratory testing and issues certificates. the clia database, updated monthly, lists. Laboratory Clia Lookup.
From www.collaborativetherapeutics.com
Ultimate CLIA Lab Building Checklist Laboratory Clia Lookup The clia lookup by npi or clia npi. the clia program sets standards for clinical laboratory testing and issues certificates. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. enter any combination of fields and select search. cdc. Laboratory Clia Lookup.
From haluxdiagnostic.com
What are CLIAwaived drug tests and why are they important? Laboratory Clia Lookup on january 16, 2024, cdc retired its clia laboratory search tool. the clia program sets standards for clinical laboratory testing and issues certificates. enter any combination of fields and select search. You can use the analyte drop down box to select a. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that. Laboratory Clia Lookup.
From www.laboratoryeconomics.com
The CLIA Compliance Reference Guide for Laboratory Managers LABORATORY ECONOMiCS Laboratory Clia Lookup enter any combination of fields and select search. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. The clia. Laboratory Clia Lookup.
From www.collaborativetherapeutics.com
CLIA Application Video Tutorial + Checklist Laboratory Clia Lookup the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. clinical laboratory improvement amendments (clia) how to apply for a clia certificate,. Laboratory Clia Lookup.
From tvhcare.org
TVH Laboratory Achieves Outstanding Results in Clinical Laboratory Improvement Amendments (CLIA Laboratory Clia Lookup The clia lookup by npi or clia npi. enter any combination of fields and select search. You can use the analyte drop down box to select a. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. cdc. Laboratory Clia Lookup.
From www.youtube.com
CLIA FULLY AUTOMATIC LABORATORY MACHINE YouTube Laboratory Clia Lookup clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. The clia lookup by npi or clia npi. You can use the analyte drop down box to select a. on january 16, 2024, cdc retired its clia laboratory search tool. enter any combination of fields and select search. You can find laboratory demographic. Laboratory Clia Lookup.
From www.scribd.com
CLIA Requirements For Proficiency Testing The Basics For Laboratory Professionals PDF Laboratory Clia Lookup You can find laboratory demographic information on the. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. on january 16, 2024, cdc retired its clia laboratory search tool. enter any combination of fields and select. Laboratory Clia Lookup.
From www.mriglobal.org
Assay Testing in Our CAP/CLIA Laboratory MRIGlobal Laboratory Clia Lookup cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere to clinical. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. The clia lookup by npi or clia npi. the clia program sets standards for clinical laboratory testing and issues certificates. enter any combination of fields and. Laboratory Clia Lookup.
From trchealthcare.com
CLIA (Clinical Laboratory Improvement Amendments) and Why Pharmacies Care TRC Healthcare Laboratory Clia Lookup the clia program sets standards for clinical laboratory testing and issues certificates. clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. You can find laboratory demographic information on the. the clia database, updated monthly, lists records of all commercially. Laboratory Clia Lookup.
From www.seqmatic.com
CLIA Laboratory SeqMatic Laboratory Clia Lookup clinical laboratory improvement amendments (clia) how to apply for a clia certificate, including international. The clia lookup by npi or clia npi. enter any combination of fields and select search. You can find laboratory demographic information on the. the clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards. the clia database, updated monthly, lists. Laboratory Clia Lookup.
From www.laboratoryeconomics.com
CLIA DATABASE of 33,000 LABS LABORATORY ECONOMiCS Laboratory Clia Lookup the clia database, updated monthly, lists records of all commercially marketed laboratory tests that have been. The clia lookup by npi or clia npi. on january 16, 2024, cdc retired its clia laboratory search tool. You can find laboratory demographic information on the. cdc laboratories that perform clinical testing (except clinical trials and basic research) must adhere. Laboratory Clia Lookup.