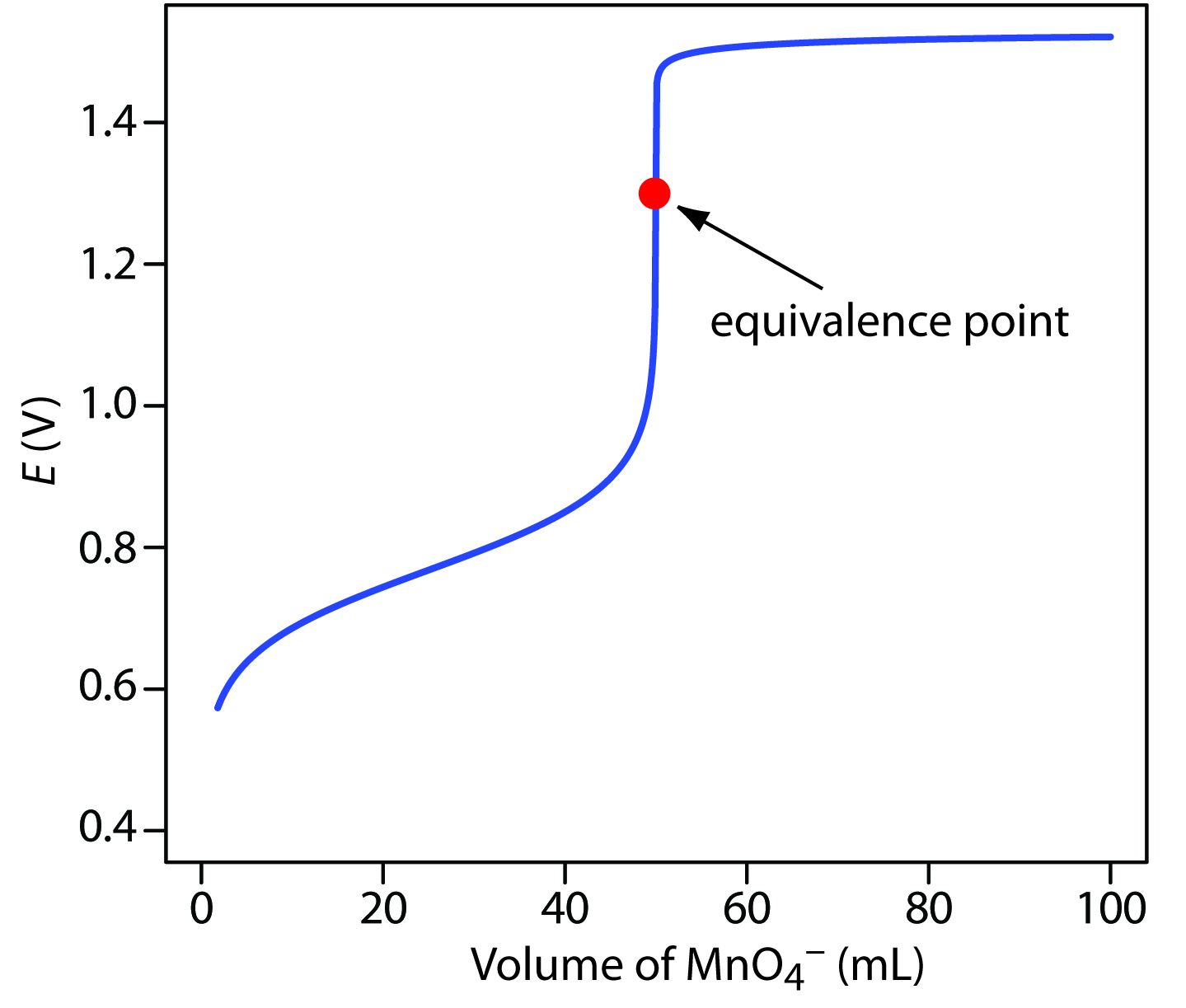
End Point In Potentiometric Titration . Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Instead, the electric potential across the substance is measured. In this method, there is no use of a chemical indicator. It discusses the basic principles of potentiometry including electrode potentials. It is used in the characterization of acids. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. This document provides information on potentiometry and potentiometric titration. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. The linearization method devised by schwartz is a powerful tool to determine titrations end point and.
from chem.libretexts.org
The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. It discusses the basic principles of potentiometry including electrode potentials. This document provides information on potentiometry and potentiometric titration. In this method, there is no use of a chemical indicator. It is used in the characterization of acids. Instead, the electric potential across the substance is measured.
9.4 Redox Titrations Chemistry LibreTexts
End Point In Potentiometric Titration It is used in the characterization of acids. It discusses the basic principles of potentiometry including electrode potentials. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Instead, the electric potential across the substance is measured. It is used in the characterization of acids. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. This document provides information on potentiometry and potentiometric titration. In this method, there is no use of a chemical indicator.
From article.sapub.org
Determination of the End Point in Potentiometric Titrations Gran and End Point In Potentiometric Titration Instead, the electric potential across the substance is measured. In this method, there is no use of a chemical indicator. It is used in the characterization of acids. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Potentiometric titration refers to a chemical method of analysis where the endpoint of. End Point In Potentiometric Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts End Point In Potentiometric Titration Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. It is used in the characterization of. End Point In Potentiometric Titration.
From mungfali.com
Endpoint Titration Curve End Point In Potentiometric Titration It discusses the basic principles of potentiometry including electrode potentials. Instead, the electric potential across the substance is measured. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. It is used in the characterization of acids. This document provides information on potentiometry and potentiometric titration. In this method, there is. End Point In Potentiometric Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax End Point In Potentiometric Titration In this method, there is no use of a chemical indicator. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. This document provides information on potentiometry and potentiometric titration. It discusses the basic principles of potentiometry including electrode potentials. Instead, the electric potential across the substance is measured. The linearization. End Point In Potentiometric Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 End Point In Potentiometric Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. This document provides information on potentiometry and potentiometric titration. In this method, there is no use of a chemical indicator. It is used in the characterization of acids. It discusses the basic principles of potentiometry including electrode potentials. The potentiometric. End Point In Potentiometric Titration.
From pubs.sciepub.com
Figure 16. Second derivative plot of the titration of chloride ion (0 End Point In Potentiometric Titration The linearization method devised by schwartz is a powerful tool to determine titrations end point and. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. In this method, there is no use of a chemical indicator. Potentiometric titration refers to a chemical method of analysis where the endpoint of the. End Point In Potentiometric Titration.
From www.youtube.com
End Point Determination by Potentiometry Titrations Pharmaceutical End Point In Potentiometric Titration This document provides information on potentiometry and potentiometric titration. Instead, the electric potential across the substance is measured. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. It discusses the basic principles of potentiometry including. End Point In Potentiometric Titration.
From opentextbc.ca
14.7 AcidBase Titrations Chemistry End Point In Potentiometric Titration This document provides information on potentiometry and potentiometric titration. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. It discusses the basic principles of potentiometry including electrode potentials. The linearization. End Point In Potentiometric Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts End Point In Potentiometric Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. This document provides information on potentiometry and potentiometric titration. In this method, there is no use of a chemical indicator. Instead, the electric potential across the substance is measured. It discusses the basic principles of potentiometry including electrode potentials. Potentiometric. End Point In Potentiometric Titration.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki End Point In Potentiometric Titration Instead, the electric potential across the substance is measured. It discusses the basic principles of potentiometry including electrode potentials. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. It is used in the characterization of acids. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. This document provides. End Point In Potentiometric Titration.
From www.youtube.com
Methods to determine end point of potentiometric titration End Point In Potentiometric Titration It discusses the basic principles of potentiometry including electrode potentials. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Instead, the electric potential across the substance is measured. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. In this method, there is no use of a chemical indicator.. End Point In Potentiometric Titration.
From modernalternativemama.com
Potentiometric Acid Base Titration End Point In Potentiometric Titration The linearization method devised by schwartz is a powerful tool to determine titrations end point and. In this method, there is no use of a chemical indicator. This document provides information on potentiometry and potentiometric titration. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Potentiometric titration refers to a. End Point In Potentiometric Titration.
From en.ppt-online.org
Electroanalytical Chemistry online presentation End Point In Potentiometric Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Instead, the electric potential across the substance is measured. In. End Point In Potentiometric Titration.
From www.animalia-life.club
Endpoint Titration End Point In Potentiometric Titration Instead, the electric potential across the substance is measured. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. It discusses the basic principles of potentiometry including electrode potentials. The linearization method devised by schwartz is a. End Point In Potentiometric Titration.
From www.slideserve.com
PPT POTENTIOMETRY 9 th lecture PowerPoint Presentation, free download End Point In Potentiometric Titration It discusses the basic principles of potentiometry including electrode potentials. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. Instead,. End Point In Potentiometric Titration.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID6718194 End Point In Potentiometric Titration Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. It is used in the characterization of acids. In this method, there is no use of a chemical indicator. The linearization method devised by schwartz is a. End Point In Potentiometric Titration.
From www.youtube.com
methods to determine end point of potentiometric titration End Point In Potentiometric Titration It is used in the characterization of acids. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. This document provides information on potentiometry and potentiometric titration. It discusses the basic principles of potentiometry including electrode potentials. Potentiometric titration refers. End Point In Potentiometric Titration.
From www.youtube.com
Potentiometric acid base titrations YouTube End Point In Potentiometric Titration This document provides information on potentiometry and potentiometric titration. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Instead, the electric potential across the substance is measured. Potentiometric titration is. End Point In Potentiometric Titration.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID4072119 End Point In Potentiometric Titration The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Instead, the electric potential across the substance is measured. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. It is used in the characterization of acids. Potentiometric titration is a. End Point In Potentiometric Titration.
From www.youtube.com
Methods for Determining end point in potentiometric titration ll End Point In Potentiometric Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. In this method, there is no use of a chemical indicator. The linearization method devised by schwartz is a powerful tool. End Point In Potentiometric Titration.
From www.slideserve.com
PPT TITRATION CURVES PowerPoint Presentation ID1130069 End Point In Potentiometric Titration It discusses the basic principles of potentiometry including electrode potentials. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. In this method, there is no use of a chemical indicator. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Potentiometric titration refers to a chemical method of analysis. End Point In Potentiometric Titration.
From www.animalia-life.club
Endpoint Titration End Point In Potentiometric Titration It is used in the characterization of acids. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. In this method, there is no use of a chemical indicator. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The linearization method devised by schwartz is a. End Point In Potentiometric Titration.
From www.studyread.com
Potentiometric Titration with Its Principle, Applications and Advanatages End Point In Potentiometric Titration It is used in the characterization of acids. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. It discusses the basic principles of potentiometry including electrode potentials. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The linearization method devised by schwartz is a powerful. End Point In Potentiometric Titration.
From www.researchgate.net
Potentiometric titration curves for 0.5 mM phthalic acid, 0.5 mM UO End Point In Potentiometric Titration The linearization method devised by schwartz is a powerful tool to determine titrations end point and. This document provides information on potentiometry and potentiometric titration. Instead, the electric potential across the substance is measured. In this method, there is no use of a chemical indicator. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric. End Point In Potentiometric Titration.
From article.sapub.org
Determination of the End Point in Potentiometric Titrations Gran and End Point In Potentiometric Titration The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. Potentiometric titration refers to a chemical method of analysis where the. End Point In Potentiometric Titration.
From article.sapub.org
Determination of the End Point in Potentiometric Titrations Gran and End Point In Potentiometric Titration It discusses the basic principles of potentiometry including electrode potentials. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. It is used in the characterization of acids. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The potentiometric titrations are particularly useful where visual. End Point In Potentiometric Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts End Point In Potentiometric Titration It discusses the basic principles of potentiometry including electrode potentials. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. The potentiometric titrations are particularly useful where visual end point detection (in the normal. End Point In Potentiometric Titration.
From www.researchgate.net
A typical high resolution potentiometric titration plot (Fe(II) vs End Point In Potentiometric Titration Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Instead, the electric potential across the substance is measured. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. It is used in the characterization of acids. In this method, there is no use of a chemical. End Point In Potentiometric Titration.
From pubs.rsc.org
A rapid method of forecasting the endpoint in potentiometric End Point In Potentiometric Titration It discusses the basic principles of potentiometry including electrode potentials. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. In this method, there is no use of a chemical indicator. It is used in the characterization of acids. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. This. End Point In Potentiometric Titration.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 End Point In Potentiometric Titration The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. In this method, there is no use of a chemical indicator. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is. End Point In Potentiometric Titration.
From mavink.com
Potentiometric Titration Curve End Point In Potentiometric Titration The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Instead, the electric potential across the substance is measured. In this method, there is no use of a chemical indicator. This document provides information on potentiometry and potentiometric titration. It is used in the characterization of acids. The linearization method devised. End Point In Potentiometric Titration.
From studylib.net
2 POTENTIOMETRIC TITRATIONS End Point In Potentiometric Titration Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. It is used in the characterization of acids. Instead, the electric potential across the substance is measured. It discusses the basic principles of potentiometry including electrode. End Point In Potentiometric Titration.
From www.youtube.com
POTENTIOMETRIC TITRATIONS YouTube End Point In Potentiometric Titration It is used in the characterization of acids. The potentiometric titrations are particularly useful where visual end point detection (in the normal volumetric titration) is dicult in. Instead, the electric potential across the substance is measured. In this method, there is no use of a chemical indicator. Potentiometric titration refers to a chemical method of analysis where the endpoint of. End Point In Potentiometric Titration.
From www.studypool.com
SOLUTION Principle of potentiometric titration end point determination End Point In Potentiometric Titration The linearization method devised by schwartz is a powerful tool to determine titrations end point and. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. Instead, the electric potential across the substance is measured. It is used in the characterization of acids. This document provides information on potentiometry and. End Point In Potentiometric Titration.
From www.youtube.com
Method for determination of end point of potentiometric titration End Point In Potentiometric Titration Instead, the electric potential across the substance is measured. The linearization method devised by schwartz is a powerful tool to determine titrations end point and. Potentiometric titration is a laboratory method to determine the concentration of a given analyte. Potentiometric titration refers to a chemical method of analysis where the endpoint of the titration is monitored using an indicator. This. End Point In Potentiometric Titration.