Copper Ii Nitrate Positive And Negative Ion . — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how to name ionic compounds using a systematic approach based on iupac guidelines.
from www.alamy.com
— learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. learn how to name ionic compounds using a systematic approach based on iupac guidelines. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing.
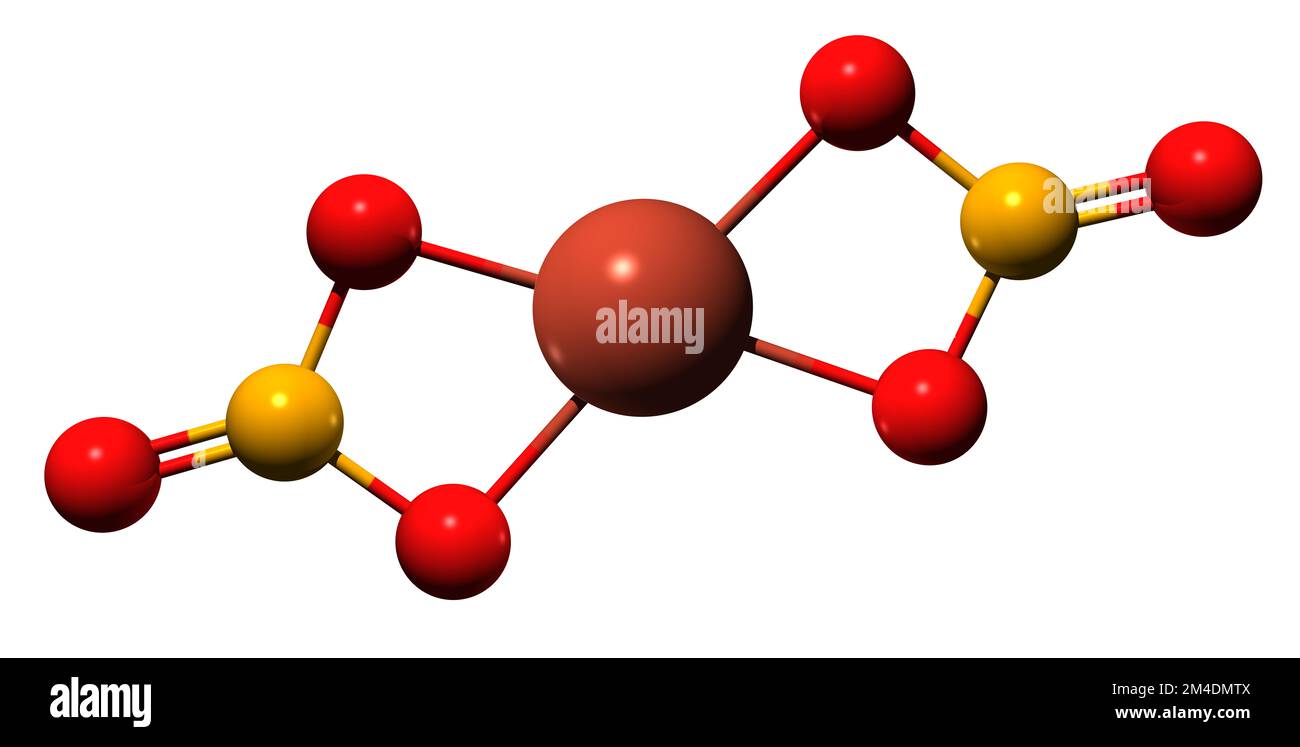
3D image of Copper II nitrate skeletal formula molecular chemical
Copper Ii Nitrate Positive And Negative Ion — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn how to name ionic compounds using a systematic approach based on iupac guidelines. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii Nitrate Positive And Negative Ion the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at. Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
CHEMICAL Reaction .Copper ii Nitrate and Ammonium Hydroxide. YouTube Copper Ii Nitrate Positive And Negative Ion learn how to name ionic compounds using a systematic approach based on iupac guidelines. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. with ph near neutral (ph = 7),. Copper Ii Nitrate Positive And Negative Ion.
From sea-hill.co.th
QREC COPPER (II) NITRATE TRIHYDRATE seahill.co.th Copper Ii Nitrate Positive And Negative Ion learn how to name ionic compounds using a systematic approach based on iupac guidelines. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how copper(ii) ions are. Copper Ii Nitrate Positive And Negative Ion.
From www.chegg.com
Solved 3. Copper (II) nitrate + Sodium phosphate Molecular Copper Ii Nitrate Positive And Negative Ion learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to. Copper Ii Nitrate Positive And Negative Ion.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Copper Ii Nitrate Positive And Negative Ion the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. learn how to name ionic compounds using a systematic approach based on iupac guidelines. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how copper(ii) ions are. Copper Ii Nitrate Positive And Negative Ion.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Copper Ii Nitrate Positive And Negative Ion learn how to name ionic compounds using a systematic approach based on iupac guidelines. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution.. Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii Nitrate Positive And Negative Ion with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same. Copper Ii Nitrate Positive And Negative Ion.
From lab.honeywell.com
Copper(II) nitrate trihydrate 61194 Honeywell Research Chemicals Copper Ii Nitrate Positive And Negative Ion learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. with ph near neutral (ph = 7), the potential of the nitrate reduction half. Copper Ii Nitrate Positive And Negative Ion.
From www.carolina.com
Copper (II) Nitrate Solution, 0.1 M, Laboratory Grade, 500 mL Copper Ii Nitrate Positive And Negative Ion learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how to name ionic compounds using a systematic approach based on iupac guidelines. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. learn how copper(ii) ions are. Copper Ii Nitrate Positive And Negative Ion.
From www.sciencephoto.com
Copper(II) nitrate Stock Image C043/2653 Science Photo Library Copper Ii Nitrate Positive And Negative Ion learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. with ph. Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
HOW TO MAKE COPPER (II) NITRATE YouTube Copper Ii Nitrate Positive And Negative Ion with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how to name ionic compounds using a systematic approach based on iupac guidelines.. Copper Ii Nitrate Positive And Negative Ion.
From courses.lumenlearning.com
3.4 Ionic Nomenclature The Basics of General, Organic, and Biological Copper Ii Nitrate Positive And Negative Ion the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. learn how to name ionic compounds using a systematic approach based on iupac guidelines. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how copper(ii) ions are. Copper Ii Nitrate Positive And Negative Ion.
From www.numerade.com
SOLVED a) Why are there two nitrate ions in the structure of copper(II Copper Ii Nitrate Positive And Negative Ion — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for. Copper Ii Nitrate Positive And Negative Ion.
From www.ravichemindustries.net
Copper Nitrate in Vadodara, India from Ravi Chem Industries Copper Ii Nitrate Positive And Negative Ion learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops. Copper Ii Nitrate Positive And Negative Ion.
From www.numerade.com
SOLVED 1. When copper(II) chloride reacts with sodium nitrate, copper Copper Ii Nitrate Positive And Negative Ion learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. learn how to name ionic compounds using a systematic approach based on iupac guidelines. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts. Copper Ii Nitrate Positive And Negative Ion.
From www.thermofisher.com
Copper(II) nitrate trihydrate, 99, pure, Thermo Scientific™ Copper Ii Nitrate Positive And Negative Ion learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. — learn how to test for positively charged ions using sodium hydroxide solution, flame. Copper Ii Nitrate Positive And Negative Ion.
From www.chegg.com
Solved When Copper (II) Chloride Reacts With Sodium Nitra... Copper Ii Nitrate Positive And Negative Ion the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell. Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium Copper Ii Nitrate Positive And Negative Ion with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how copper(ii) ions are deposited at the cathode and oxygen is given off. Copper Ii Nitrate Positive And Negative Ion.
From pubs.rsc.org
Spectroscopy and photochemistry of copper nitrate clusters Physical Copper Ii Nitrate Positive And Negative Ion learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops. Copper Ii Nitrate Positive And Negative Ion.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Ii Nitrate Positive And Negative Ion the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive. Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
How to Balance Cu(NO3)2 = CuO + NO2 + O2 Copper (II) nitrate Copper Ii Nitrate Positive And Negative Ion — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and. Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
Nitrate Ion Lewis Structure How to Draw the Lewis Structure for Copper Ii Nitrate Positive And Negative Ion learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. learn how to name ionic compounds using a systematic approach based on iupac guidelines. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. with ph near neutral (ph =. Copper Ii Nitrate Positive And Negative Ion.
From www.chegg.com
Solved 2. Formulas of ionic compounds Name Positive Ion Copper Ii Nitrate Positive And Negative Ion learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. learn how to name ionic compounds using a systematic approach based on iupac guidelines. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn. Copper Ii Nitrate Positive And Negative Ion.
From www.alamy.com
3D image of Copper II nitrate skeletal formula molecular chemical Copper Ii Nitrate Positive And Negative Ion the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn how. Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
How to Write the Net Ionic Equation for K2S + Cu(NO3)2 = KNO3 + CuS Copper Ii Nitrate Positive And Negative Ion — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and. Copper Ii Nitrate Positive And Negative Ion.
From brainly.in
Name all groups like hydroxide group,sulphate group,nitrate group, and Copper Ii Nitrate Positive And Negative Ion learn how to name ionic compounds using a systematic approach based on iupac guidelines. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. with. Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Nitrate Positive And Negative Ion with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. the rule for constructing formulas for ionic compounds containing. Copper Ii Nitrate Positive And Negative Ion.
From www.pinterest.com
Copper (II) Nitrate Trihydrate, 500g LabGrade The Curated Chemical Copper Ii Nitrate Positive And Negative Ion learn how to name ionic compounds using a systematic approach based on iupac guidelines. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn. Copper Ii Nitrate Positive And Negative Ion.
From www.t3db.ca
T3DB Copper(II) nitrate Copper Ii Nitrate Positive And Negative Ion learn how to name ionic compounds using a systematic approach based on iupac guidelines. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. . Copper Ii Nitrate Positive And Negative Ion.
From testbook.com
Copper (II) Nitrate Formula Learn Structure, Properties, Uses Copper Ii Nitrate Positive And Negative Ion learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. learn how to write chemical formulas for ionic compounds, which are composed of alternating positive. Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
Copper metal reacts with nitric acid to form a solution of copper(II Copper Ii Nitrate Positive And Negative Ion learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. learn how to name ionic compounds using a systematic. Copper Ii Nitrate Positive And Negative Ion.
From www.numerade.com
SOLVED Give the complete ionic equation for the reaction (if any) that Copper Ii Nitrate Positive And Negative Ion learn how to write chemical formulas for ionic compounds, which are composed of alternating positive and negative ions in. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of. Copper Ii Nitrate Positive And Negative Ion.
From www.ck12.org
Ionic Compounds CK12 Foundation Copper Ii Nitrate Positive And Negative Ion learn how to name ionic compounds using a systematic approach based on iupac guidelines. learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. the rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing. . Copper Ii Nitrate Positive And Negative Ion.
From www.youtube.com
Write the chemical formula of Copper nitrate YouTube Copper Ii Nitrate Positive And Negative Ion learn how copper(ii) ions are deposited at the cathode and oxygen is given off at the anode in the electrolysis of copper(ii) sulphate solution. with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. the rule for constructing formulas for ionic compounds containing. Copper Ii Nitrate Positive And Negative Ion.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry Copper Ii Nitrate Positive And Negative Ion with ph near neutral (ph = 7), the potential of the nitrate reduction half cell drops from +0.96 volts to +0.408, so then the. learn how to name ionic compounds using a systematic approach based on iupac guidelines. — learn how to test for positively charged ions using sodium hydroxide solution, flame tests and other. the. Copper Ii Nitrate Positive And Negative Ion.