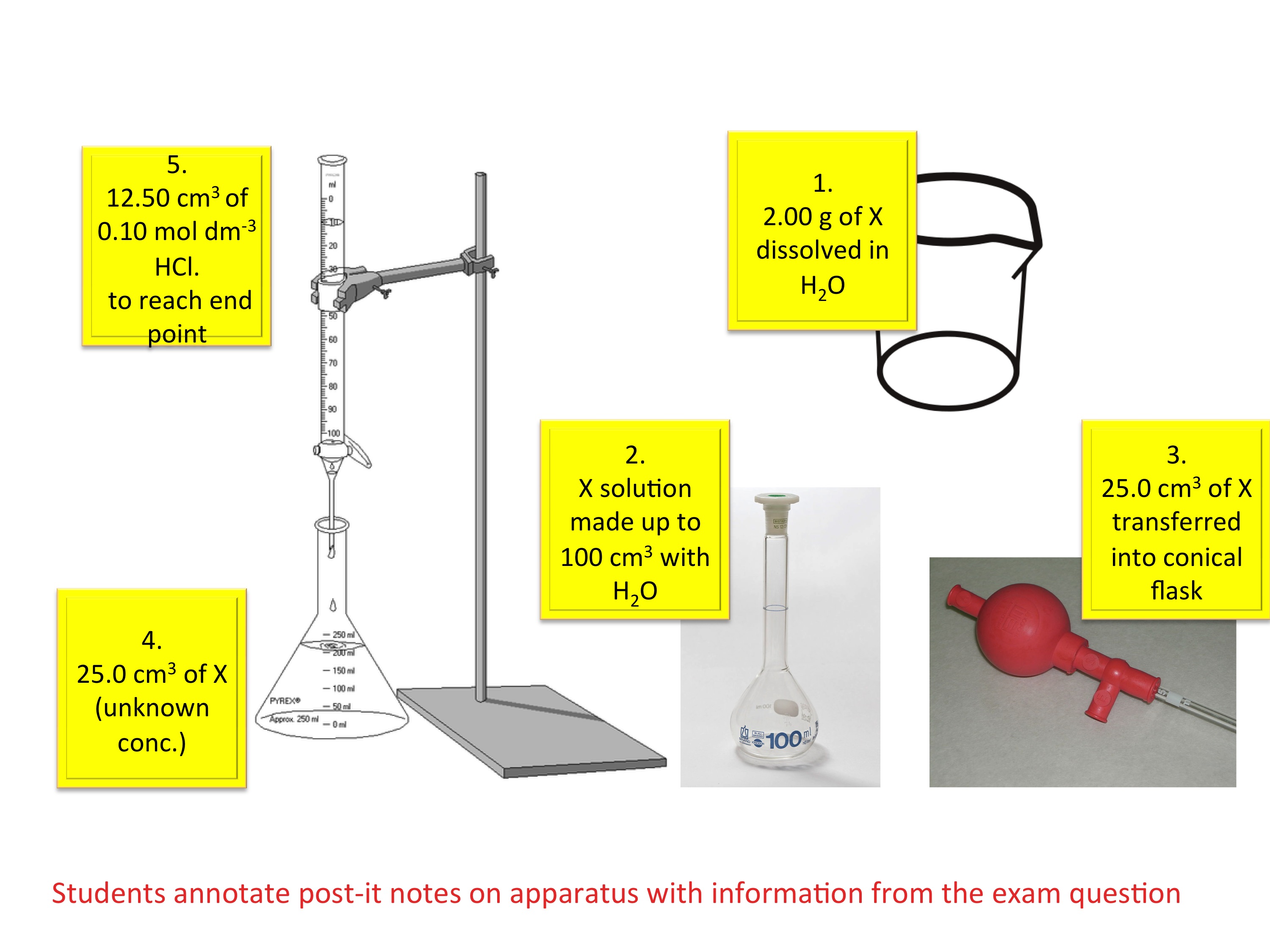
How To Do Titration Problems . then you are in the right place! How to do titration calculations // hsc chemistry. A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) It provides a basic introduction. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. By the end of this section, you will be able to: this chemistry video tutorial explains how to solve acid base titration problems. this video walks through common examples of calculation problems for titration. to do volumetric analysis, we use a method called titration which involves:
from thescienceteacher.co.uk
A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. this video walks through common examples of calculation problems for titration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. By the end of this section, you will be able to: A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) How to do titration calculations // hsc chemistry. It provides a basic introduction. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. then you are in the right place!
Concentration and titrations teaching resources the science teacher
How To Do Titration Problems the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. then you are in the right place! How to do titration calculations // hsc chemistry. to do volumetric analysis, we use a method called titration which involves: A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) It provides a basic introduction. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. this chemistry video tutorial explains how to solve acid base titration problems. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. this video walks through common examples of calculation problems for titration. By the end of this section, you will be able to:
From exyfoiyjs.blob.core.windows.net
Titration Reaction Problems at Franklyn Haynes blog How To Do Titration Problems It provides a basic introduction. this chemistry video tutorial explains how to solve acid base titration problems. A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. How to do titration calculations. How To Do Titration Problems.
From tukioka-clinic.com
️ Acid base titration problems with answers pdf. Eleventh grade Lesson How To Do Titration Problems A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. By the end of this section, you will be able to: this chemistry video tutorial explains how to solve acid base titration problems. In this tutorial, you will find information on titration, including the chemicals that are commonly used and. How To Do Titration Problems.
From cesdjxor.blob.core.windows.net
Titration Neutralization Equation at Staci Arroyo blog How To Do Titration Problems this video walks through common examples of calculation problems for titration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. this chemistry video tutorial explains how to solve acid base titration problems. then you are in the right place! A solution of unknown concentration (analyte. How To Do Titration Problems.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Do Titration Problems to do volumetric analysis, we use a method called titration which involves: In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. It provides a basic introduction. this chemistry. How To Do Titration Problems.
From studylib.net
Titration Problems How To Do Titration Problems In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. this chemistry video tutorial explains how to solve acid base titration problems. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this section, we will see how. How To Do Titration Problems.
From classburns.z4.web.core.windows.net
Titration Practical Questions And Answers How To Do Titration Problems How to do titration calculations // hsc chemistry. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. this video walks through common examples of calculation problems for titration. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. then you. How To Do Titration Problems.
From giogrwbcc.blob.core.windows.net
Acidity Titration Formula at Kimberly Carroll blog How To Do Titration Problems this chemistry video tutorial explains how to solve acid base titration problems. A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) then you are in the right place! It provides a basic introduction. By the end of this section, you will be able to: How to do titration calculations. How To Do Titration Problems.
From fyomgpmqq.blob.core.windows.net
Titration Calculations Exam Questions at Bree Duckett blog How To Do Titration Problems the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. It provides a basic introduction. How to do titration calculations //. How To Do Titration Problems.
From fyoadtmij.blob.core.windows.net
Redox Indicators In Chemistry at Julia Anderson blog How To Do Titration Problems A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. then you are in the right place! this video walks through common examples of calculation problems for titration.. How To Do Titration Problems.
From www.youtube.com
Back Titration Example YouTube How To Do Titration Problems By the end of this section, you will be able to: to do volumetric analysis, we use a method called titration which involves: A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) this chemistry video tutorial explains how to solve acid base titration problems. How to do titration calculations. How To Do Titration Problems.
From cesdjxor.blob.core.windows.net
Titration Neutralization Equation at Staci Arroyo blog How To Do Titration Problems this chemistry video tutorial explains how to solve acid base titration problems. this video walks through common examples of calculation problems for titration. then you are in the right place! In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. the above equation works only for neutralizations in. How To Do Titration Problems.
From gioltgugv.blob.core.windows.net
Titration Lab Problems at Dorothy Stephenson blog How To Do Titration Problems the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. How to do titration calculations // hsc chemistry. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. to do volumetric analysis, we use a method called titration which involves:. How To Do Titration Problems.
From www.chegg.com
Solved To review how titration calculations work, here's a How To Do Titration Problems How to do titration calculations // hsc chemistry. It provides a basic introduction. this chemistry video tutorial explains how to solve acid base titration problems. to do volumetric analysis, we use a method called titration which involves: in this section, we will see how to perform calculations to predict the ph at any point in a titration. How To Do Titration Problems.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube How To Do Titration Problems In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. How to do titration calculations // hsc chemistry. It provides a basic introduction. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A 25 ml solution of 0.5 m naoh. How To Do Titration Problems.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 How To Do Titration Problems this video walks through common examples of calculation problems for titration. A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. then you are in the right place! to do. How To Do Titration Problems.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube How To Do Titration Problems A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) to do volumetric analysis, we use a method called titration which involves: In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. How to do titration calculations // hsc chemistry. this chemistry. How To Do Titration Problems.
From gioltgugv.blob.core.windows.net
Titration Lab Problems at Dorothy Stephenson blog How To Do Titration Problems How to do titration calculations // hsc chemistry. then you are in the right place! It provides a basic introduction. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. to do volumetric analysis, we use a method called titration which involves: By the end of this. How To Do Titration Problems.
From www.tffn.net
Solving Titration Problems A StepbyStep Guide The Enlightened Mindset How To Do Titration Problems A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. It provides a basic introduction. By the end of this section, you will be able to: then you are in the right place! this chemistry video tutorial explains how to solve acid base titration problems. the above equation. How To Do Titration Problems.
From exyqynbtm.blob.core.windows.net
Titration Experiment Class 11 Readings at Caleb Heath blog How To Do Titration Problems then you are in the right place! It provides a basic introduction. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. How to do titration calculations // hsc chemistry. to do volumetric analysis, we use a method called titration which involves: A 25 ml solution of 0.5 m naoh. How To Do Titration Problems.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube How To Do Titration Problems How to do titration calculations // hsc chemistry. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. this chemistry video tutorial explains how to solve acid base titration problems. to. How To Do Titration Problems.
From tukioka-clinic.com
👍 Solve titration problems. How to solve titration problems step by How To Do Titration Problems this chemistry video tutorial explains how to solve acid base titration problems. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. It provides a basic introduction. this video walks through. How To Do Titration Problems.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher How To Do Titration Problems A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. this chemistry video tutorial explains how to solve acid base titration problems. the above equation works only for neutralizations in which. How To Do Titration Problems.
From worksheetcampusslewed.z21.web.core.windows.net
Chemistry Titration Questions And Answers How To Do Titration Problems to do volumetric analysis, we use a method called titration which involves: How to do titration calculations // hsc chemistry. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. It provides a basic introduction. then you are in the. How To Do Titration Problems.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations How To Do Titration Problems A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. It provides a basic introduction. then you are in the right place! to do volumetric analysis, we use a method called. How To Do Titration Problems.
From exyfxaqfh.blob.core.windows.net
Titration Practice Problems With Answers Pdf at Jessica Frazier blog How To Do Titration Problems By the end of this section, you will be able to: the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. A 25 ml solution of 0.5 m naoh is titrated. How To Do Titration Problems.
From www.vrogue.co
Acid Base Titrations · Chemistry Titration Curves Equivalence Point How To Do Titration Problems this video walks through common examples of calculation problems for titration. to do volumetric analysis, we use a method called titration which involves: A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in. How To Do Titration Problems.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145706 How To Do Titration Problems then you are in the right place! By the end of this section, you will be able to: this chemistry video tutorial explains how to solve acid base titration problems. this video walks through common examples of calculation problems for titration. to do volumetric analysis, we use a method called titration which involves: the above. How To Do Titration Problems.
From exyyqmwga.blob.core.windows.net
Titration Hazards at Scott blog How To Do Titration Problems How to do titration calculations // hsc chemistry. A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) this chemistry video tutorial explains how to solve acid base titration problems. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.. How To Do Titration Problems.
From www.microlit.us
Uses of Burettes in Pharmaceutical Industry Diagrams & Functions How To Do Titration Problems this video walks through common examples of calculation problems for titration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. It provides a basic introduction. in this section, we will see how to perform calculations to predict the ph at any point in a titration of. How To Do Titration Problems.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions How To Do Titration Problems By the end of this section, you will be able to: A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. In this. How To Do Titration Problems.
From dxolueths.blob.core.windows.net
Titration Graph Weak Acid Strong Base at Rebecca Baldridge blog How To Do Titration Problems this chemistry video tutorial explains how to solve acid base titration problems. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the. By the end of this section, you will be able. How To Do Titration Problems.
From ceplknbb.blob.core.windows.net
Acid Base Titration Questions at Deborah Wedel blog How To Do Titration Problems By the end of this section, you will be able to: then you are in the right place! this video walks through common examples of calculation problems for titration. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. . How To Do Titration Problems.
From fyoczrqcn.blob.core.windows.net
How To Do The Calculations For A Titration at David McClendon blog How To Do Titration Problems in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. to do volumetric analysis, we use a method called titration which involves: It provides a basic introduction. this video walks through common examples of calculation problems for titration. the. How To Do Titration Problems.
From studylib.net
Titration Practice Worksheet How To Do Titration Problems A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. then you are in the right place! How to do titration calculations // hsc chemistry. A solution of unknown concentration (analyte in conical flask) a solution of known concentration (titrant in the burette) In this tutorial, you will find information. How To Do Titration Problems.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets How To Do Titration Problems How to do titration calculations // hsc chemistry. then you are in the right place! to do volumetric analysis, we use a method called titration which involves: A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. this chemistry video tutorial explains how to solve acid base titration. How To Do Titration Problems.