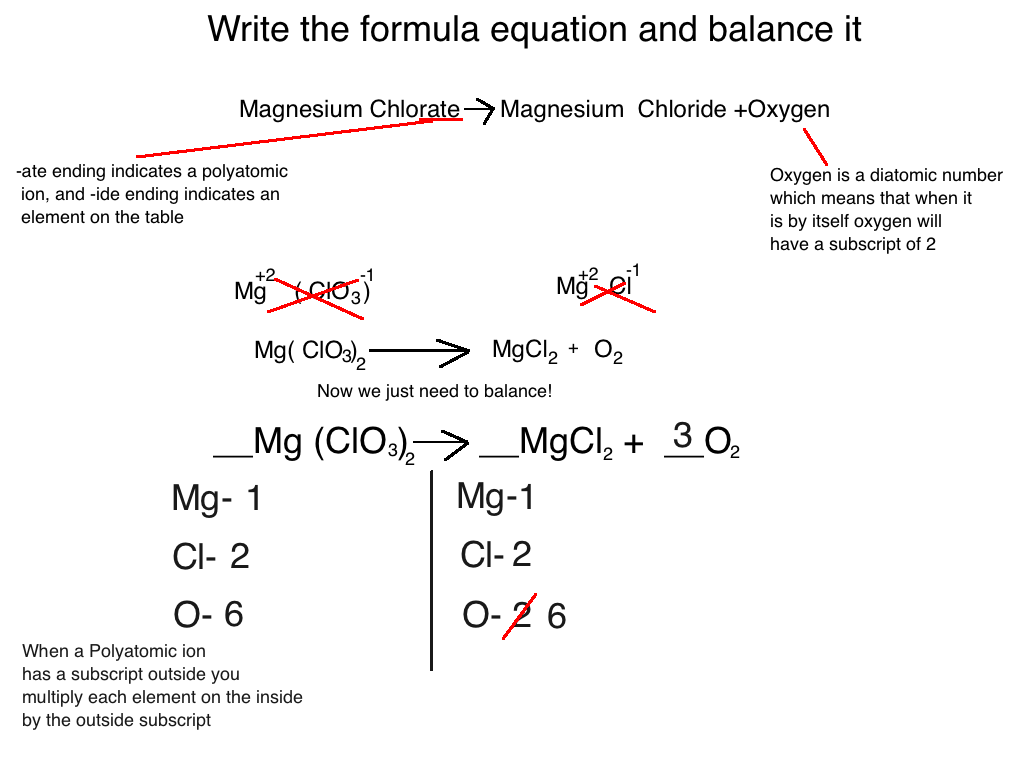
Balance Charge By Adding Electrons . Add electrons to balance out any excess charge so that each side has an equal charge. Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons.
from chemistry101efhs.weebly.com
Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Add electrons to balance out any excess charge so that each side has an equal charge.
Balancing Equations Chemistry 101
Balance Charge By Adding Electrons Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Add electrons to balance out any excess charge so that each side has an equal charge. Balance charge by adding electrons.
From slidetodoc.com
BALANCING REDOX EQUATIONS USING THE HALF EQUATION METHOD Balance Charge By Adding Electrons Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Add electrons to balance out any excess charge so that each side has an equal charge. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT Electrochemistry Lesson 5 Balancing Half Reactions PowerPoint Presentation ID4573125 Balance Charge By Adding Electrons Balance charge by adding electrons. Add electrons to balance out any excess charge so that each side has an equal charge. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance. Balance Charge By Adding Electrons.
From www.masterorganicchemistry.com
7 Factors That Stabilize Positive Charge in Organic Chemistry Master Organic Chemistry Balance Charge By Adding Electrons Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance charge by adding electrons. Add electrons to balance out any. Balance Charge By Adding Electrons.
From www.youtube.com
1.3.2 Balance equation (ionelectron method) YouTube Balance Charge By Adding Electrons Add electrons to balance out any excess charge so that each side has an equal charge. Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the. Balance Charge By Adding Electrons.
From slideplayer.com
REDOX REACTIONS REACTIONS Day 1 Review Batteries. ppt download Balance Charge By Adding Electrons Add electrons to balance out any excess charge so that each side has an equal charge. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \. Balance Charge By Adding Electrons.
From www.youtube.com
Balancing Chemical Equations YouTube Balance Charge By Adding Electrons Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Add electrons to balance out any. Balance Charge By Adding Electrons.
From studyqueries.com
Charge Of Proton Definition, Mass & More Balance Charge By Adding Electrons Add electrons to balance out any excess charge so that each side has an equal charge. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the. Balance Charge By Adding Electrons.
From slideplayer.com
Ion Electron Method Ch ppt download Balance Charge By Adding Electrons Add electrons to balance out any excess charge so that each side has an equal charge. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve. Balance Charge By Adding Electrons.
From www.youtube.com
balancing a redox reaction / oxidation number method YouTube Balance Charge By Adding Electrons Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Add electrons to balance out any excess charge so that each side has an equal charge. Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT Balancing Redox Equations PowerPoint Presentation, free download ID3469664 Balance Charge By Adding Electrons Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Balance charge by adding electrons. Add electrons to balance out any excess charge so that each side has an equal charge. Balance the charges by adding electrons (e⁻) since redox reactions involve. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT Chapter 6 OxidationReduction Reactions PowerPoint Presentation ID5980544 Balance Charge By Adding Electrons Balance charge by adding electrons. Add electrons to balance out any excess charge so that each side has an equal charge. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT Balancing Redox Equations following the electrons PowerPoint Presentation ID369776 Balance Charge By Adding Electrons Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Add electrons to balance out any. Balance Charge By Adding Electrons.
From periodictable.me
What are the Difference Between Charge and Electron? Dynamic Periodic Table of Elements and Balance Charge By Adding Electrons Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Add electrons to balance out any excess charge so that each side has an equal charge. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT RedOx Chemistry PowerPoint Presentation, free download ID5411768 Balance Charge By Adding Electrons Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Add electrons to balance out any. Balance Charge By Adding Electrons.
From www.toppr.com
WA MITTU Balancing with respect to hydrogen by adding 8H* on L.H.S., MnO4 + 8H+ + Mn++ Balance Charge By Adding Electrons Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Add electrons to balance out any excess charge so that each side has an equal charge. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the. Balance Charge By Adding Electrons.
From slidetodoc.com
Chapter 5 OxidationReduction Reactions Chemistry The Molecular Nature Balance Charge By Adding Electrons Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Add electrons to balance out any excess charge so that each side has an equal charge. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance. Balance Charge By Adding Electrons.
From www.youtube.com
Cl2=ClO3^+Cl^ Balance the chemical equation by ion electron method or half reaction method Balance Charge By Adding Electrons Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Add electrons to balance out any. Balance Charge By Adding Electrons.
From www.numerade.com
SOLVED Balance the following halfreactions by adding the appropriate number of electrons (eâ Balance Charge By Adding Electrons Balance charge by adding electrons. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Add electrons to balance out any. Balance Charge By Adding Electrons.
From courses.lumenlearning.com
Nuclear Equations Chemistry Balance Charge By Adding Electrons Add electrons to balance out any excess charge so that each side has an equal charge. Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the. Balance Charge By Adding Electrons.
From slidetodoc.com
OILRIG Oxidation is loss of electrons Reduction is Balance Charge By Adding Electrons Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Add electrons to balance out any excess charge so that each side has an equal charge. Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT Balancing Redox Equations PowerPoint Presentation, free download ID3469664 Balance Charge By Adding Electrons Balance charge by adding electrons. Add electrons to balance out any excess charge so that each side has an equal charge. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance. Balance Charge By Adding Electrons.
From slideplayer.com
OXIDATION AND REDUCTION ppt download Balance Charge By Adding Electrons Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Add electrons to balance out any excess charge so that each side has an equal charge. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance. Balance Charge By Adding Electrons.
From slideplayer.com
Ion Electron Method Ch ppt download Balance Charge By Adding Electrons Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Add electrons to balance out any excess charge so that each. Balance Charge By Adding Electrons.
From mungfali.com
Charge Balance Equation Balance Charge By Adding Electrons Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Add electrons to balance out any excess charge so that each. Balance Charge By Adding Electrons.
From slideplayer.com
Half Reactions. ppt download Balance Charge By Adding Electrons Add electrons to balance out any excess charge so that each side has an equal charge. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both.. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Presentation ID6752860 Balance Charge By Adding Electrons Add electrons to balance out any excess charge so that each side has an equal charge. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \. Balance Charge By Adding Electrons.
From slideplayer.com
Half Reactions. ppt download Balance Charge By Adding Electrons Add electrons to balance out any excess charge so that each side has an equal charge. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the. Balance Charge By Adding Electrons.
From www.vrogue.co
Balancing Chemical Equations Overview Examples Expii vrogue.co Balance Charge By Adding Electrons Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Add electrons to balance out any excess charge so that each. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT Balancing Redox Equations following the electrons PowerPoint Presentation ID369776 Balance Charge By Adding Electrons Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Add electrons to balance out any excess charge so that each. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT Balancing redox reactions PowerPoint Presentation, free download ID4939118 Balance Charge By Adding Electrons Balance charge by adding electrons. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Add electrons to balance out any excess charge so that each side has an equal charge. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT RedOx Chemistry PowerPoint Presentation, free download ID3201551 Balance Charge By Adding Electrons Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance charge by adding electrons. Balance charge by adding electrons. Add electrons to balance out any. Balance Charge By Adding Electrons.
From chemistry101efhs.weebly.com
Balancing Equations Chemistry 101 Balance Charge By Adding Electrons Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Add electrons to balance out any. Balance Charge By Adding Electrons.
From www.slideserve.com
PPT RedOx Chemistry PowerPoint Presentation, free download ID3201551 Balance Charge By Adding Electrons Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Balance charge by adding electrons. Balance charge by adding electrons. Add electrons to balance out any. Balance Charge By Adding Electrons.
From www.numerade.com
SOLVED 4. Balance charge by adding electrons. 5. Multiply each halfreaction to balance Balance Charge By Adding Electrons Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Add electrons to balance out any excess charge so that each side has an equal charge. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both.. Balance Charge By Adding Electrons.
From www.youtube.com
V28 Charge Balance YouTube Balance Charge By Adding Electrons Balance charge by adding electrons. Balance charge by adding electrons. Balance the hydrogen atoms (including those added in step 2 to balance the oxygen atom) by adding \ (\ce {h^ {+}}\) ions to. Balance the charges by adding electrons (e⁻) since redox reactions involve the transfer of electrons, balance the overall charge on both. Add electrons to balance out any. Balance Charge By Adding Electrons.