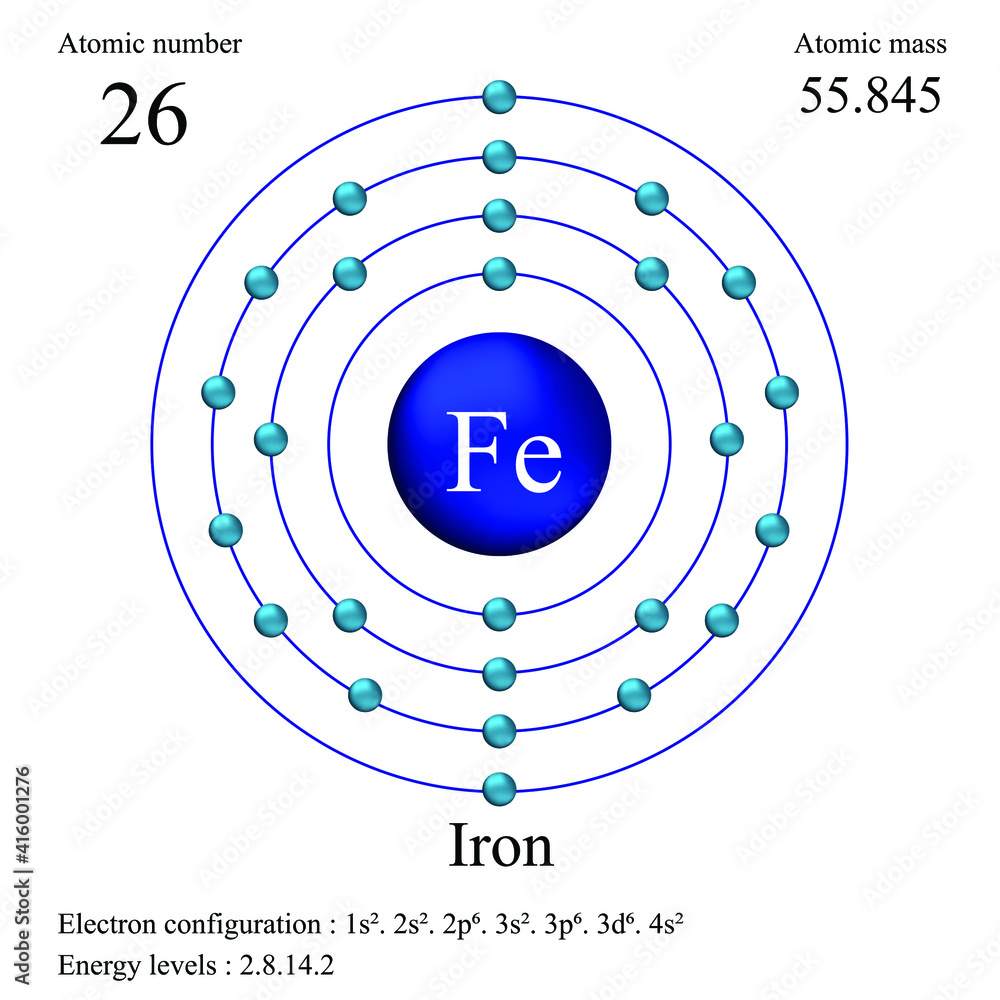
Iron Find The Atomic Mass . Determine the number of protons, neutrons, and electrons in an atom. The easiest way to find the atomic mass is to look it up on a periodic table. How to calculate atomic mass. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also. Identify the charge and relative mass of subatomic particles. The atomic mass or relative. Review the steps to find atomic mass. Define atomic and mass numbers. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. Atomic mass of iron is 55.845 u. For a single atom, atomic mass is the sum of the protons and neutrons. The atomic mass is the mass of an atom.
from stock.adobe.com
How to calculate atomic mass. Define atomic and mass numbers. Review the steps to find atomic mass. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Determine the number of protons, neutrons, and electrons in an atom. Identify the charge and relative mass of subatomic particles. For a single atom, atomic mass is the sum of the protons and neutrons. The easiest way to find the atomic mass is to look it up on a periodic table. Atomic mass of iron is 55.845 u. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also.
Iron atomic structure has atomic number, atomic mass, electron
Iron Find The Atomic Mass The atomic mass for each element is given in atomic mass units or grams per mole of atoms. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. The atomic mass is the mass of an atom. For a single atom, atomic mass is the sum of the protons and neutrons. The atomic mass or relative. Define atomic and mass numbers. Identify the charge and relative mass of subatomic particles. How to calculate atomic mass. Determine the number of protons, neutrons, and electrons in an atom. Atomic mass of iron is 55.845 u. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Review the steps to find atomic mass. The easiest way to find the atomic mass is to look it up on a periodic table. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also.
From www.slideserve.com
PPT MOLE (mol) PowerPoint Presentation, free download ID4272649 Iron Find The Atomic Mass The atomic mass for each element is given in atomic mass units or grams per mole of atoms. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each. Iron Find The Atomic Mass.
From jokermeister.weebly.com
Atomic mass of iron jokermeister Iron Find The Atomic Mass Atomic mass of iron is 55.845 u. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. Identify the charge and relative mass of subatomic particles. How to calculate atomic mass. For a single atom, atomic mass is the sum of the protons and neutrons. The easiest way to find the atomic. Iron Find The Atomic Mass.
From www.vectorstock.com
Flashcard of iron with atomic mass Royalty Free Vector Image Iron Find The Atomic Mass The atomic mass is the mass of an atom. The atomic mass or relative. How to calculate atomic mass. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Define atomic and mass numbers. Review the steps to find atomic mass. For a single. Iron Find The Atomic Mass.
From www.wikihow.com
3 Ways to Calculate Atomic Mass wikiHow Iron Find The Atomic Mass Define atomic and mass numbers. Identify the charge and relative mass of subatomic particles. For a single atom, atomic mass is the sum of the protons and neutrons. The easiest way to find the atomic mass is to look it up on a periodic table. Atomic mass of iron is 55.845 u. The atomic mass for each element is given. Iron Find The Atomic Mass.
From www.dreamstime.com
Iron Chemical Element Symbol with Atomic Mass and Atomic Number in Iron Find The Atomic Mass The easiest way to find the atomic mass is to look it up on a periodic table. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also. For a single atom, atomic mass is the sum of the protons and neutrons. The. Iron Find The Atomic Mass.
From www.numerade.com
SOLVED An iron atom has an atomic mass of 56. its atomic number is 26 Iron Find The Atomic Mass The atomic mass is the mass of an atom. The atomic mass or relative. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Define atomic and mass numbers. By calculating an average of an element’s atomic masses, weighted by the natural abundance of. Iron Find The Atomic Mass.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow Iron Find The Atomic Mass The easiest way to find the atomic mass is to look it up on a periodic table. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. Identify the charge and relative mass of subatomic particles. Atomic mass of iron is 55.845 u. Review the steps to find atomic mass. Define atomic. Iron Find The Atomic Mass.
From www.dreamstime.com
Iron Atom, with Mass and Energy Levels. Stock Vector Illustration of Iron Find The Atomic Mass Determine the number of protons, neutrons, and electrons in an atom. The atomic mass is the mass of an atom. For a single atom, atomic mass is the sum of the protons and neutrons. Review the steps to find atomic mass. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain. Iron Find The Atomic Mass.
From simm-miler.blogspot.com
Molar Mass Of Iron Molecular Mass Of All Elements In Periodic Table Iron Find The Atomic Mass The atomic mass is the mass of an atom. How to calculate atomic mass. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The easiest way to find the atomic mass is to look it up on a periodic table. The atomic mass. Iron Find The Atomic Mass.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Iron Find The Atomic Mass Review the steps to find atomic mass. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also. The atomic mass or relative. The atomic mass is the mass of an atom. The easiest way to find the atomic mass is to look. Iron Find The Atomic Mass.
From material-properties.org
Iron Periodic Table and Atomic Properties Iron Find The Atomic Mass Review the steps to find atomic mass. How to calculate atomic mass. Determine the number of protons, neutrons, and electrons in an atom. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic mass for each element is given in atomic mass. Iron Find The Atomic Mass.
From otissan.blogspot.com
Molar Mass Of Iron Calculate the molar mass of aluminum oxide (Al2O3 Iron Find The Atomic Mass Identify the charge and relative mass of subatomic particles. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. Determine the number of protons, neutrons, and electrons in an atom. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains. Iron Find The Atomic Mass.
From brainly.com
What's the mass of one iron atom in standard and scientific notation Iron Find The Atomic Mass For a single atom, atomic mass is the sum of the protons and neutrons. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also.. Iron Find The Atomic Mass.
From www.alamy.com
Fe Iron Chemical Element Periodic Table. Single vector illustration Iron Find The Atomic Mass This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Define atomic and mass numbers. The atomic mass or relative. Determine the number of protons, neutrons, and electrons in an atom. How to calculate atomic mass. The atomic mass for each element is given. Iron Find The Atomic Mass.
From www.numerade.com
SOLVED Using the average atomic mass, calculate the mass in grams of Iron Find The Atomic Mass Define atomic and mass numbers. Atomic mass of iron is 55.845 u. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. How to calculate atomic mass. Identify the charge and relative mass of subatomic particles. Determine the number of protons, neutrons, and electrons. Iron Find The Atomic Mass.
From tankhrom.weebly.com
Iron atomic number tankhrom Iron Find The Atomic Mass The easiest way to find the atomic mass is to look it up on a periodic table. The atomic mass or relative. Atomic mass of iron is 55.845 u. The atomic mass is the mass of an atom. For a single atom, atomic mass is the sum of the protons and neutrons. Identify the charge and relative mass of subatomic. Iron Find The Atomic Mass.
From www.numerade.com
SOLVED Calculate the mass of Iron(III) oxide (FezO;) that contains a Iron Find The Atomic Mass Define atomic and mass numbers. Review the steps to find atomic mass. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. Identify the charge and relative mass of subatomic particles. The easiest way to find the atomic mass is to look it up on a periodic table. The atomic mass is. Iron Find The Atomic Mass.
From www.alamy.com
Iron chemical element. Chemical symbol with atomic number and atomic Iron Find The Atomic Mass For a single atom, atomic mass is the sum of the protons and neutrons. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also. The easiest way to find the atomic mass is to look it up on a periodic table. The. Iron Find The Atomic Mass.
From byjus.com
Atomic Mass And Molecular Mass Definition Difference Mass spectrometry Iron Find The Atomic Mass The atomic mass or relative. Review the steps to find atomic mass. The atomic mass is the mass of an atom. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. How to calculate atomic mass. Identify the charge and relative mass of subatomic. Iron Find The Atomic Mass.
From slidetodoc.com
Calculating Particles for an ion Representations from the Iron Find The Atomic Mass For a single atom, atomic mass is the sum of the protons and neutrons. The atomic mass or relative. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. The easiest way to find the atomic mass is to look it up on a periodic table. Determine the number of protons, neutrons,. Iron Find The Atomic Mass.
From www.britannica.com
Iron Element, Occurrence, Uses, Properties, & Compounds Britannica Iron Find The Atomic Mass Atomic mass of iron is 55.845 u. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also. Identify the charge and relative mass of subatomic particles. The atomic mass for each element is given in atomic mass units or grams per mole. Iron Find The Atomic Mass.
From www.youtube.com
How to Find the Average Atomic Mass of Iron YouTube Iron Find The Atomic Mass This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The easiest way to find the atomic mass is to look it up on a periodic table. The atomic mass is the mass of an atom. Identify the charge and relative mass of subatomic. Iron Find The Atomic Mass.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Find The Atomic Mass This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic mass is the mass of an atom. Identify the charge and relative mass of subatomic particles. The easiest way to find the atomic mass is to look it up on a periodic. Iron Find The Atomic Mass.
From www.alamy.com
Iron chemical element. Chemical symbol with atomic number and atomic Iron Find The Atomic Mass Define atomic and mass numbers. Atomic mass of iron is 55.845 u. The atomic mass or relative. Determine the number of protons, neutrons, and electrons in an atom. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. How to calculate atomic mass. Identify. Iron Find The Atomic Mass.
From www.numerade.com
SOLVED Consider the following data for iron g 55.845 mol atomic mass Iron Find The Atomic Mass This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. How to calculate atomic mass. Atomic mass of iron is 55.845 u. For a single atom, atomic mass is the sum of the protons and neutrons. By calculating an average of an element’s atomic. Iron Find The Atomic Mass.
From ootery.weebly.com
Atomic mass of iron ootery Iron Find The Atomic Mass This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Define atomic and mass numbers. Review the steps to find atomic mass. The atomic mass or relative. The easiest way to find the atomic mass is to look it up on a periodic table.. Iron Find The Atomic Mass.
From www.nuclear-power.com
Iron Atomic Number Atomic Mass Density of Iron Iron Find The Atomic Mass Atomic mass of iron is 55.845 u. The atomic mass is the mass of an atom. The atomic mass or relative. Determine the number of protons, neutrons, and electrons in an atom. Define atomic and mass numbers. Identify the charge and relative mass of subatomic particles. The easiest way to find the atomic mass is to look it up on. Iron Find The Atomic Mass.
From www.youtube.com
Mass of iron, Fe, formed from iron oxide, Fe2O3, moles calculation Iron Find The Atomic Mass The atomic mass or relative. Define atomic and mass numbers. Determine the number of protons, neutrons, and electrons in an atom. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also. For a single atom, atomic mass is the sum of the. Iron Find The Atomic Mass.
From www.numerade.com
SOLVED Calculate the average atomic mass of iron using the isotopes in Iron Find The Atomic Mass The atomic mass is the mass of an atom. Review the steps to find atomic mass. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. The atomic mass or relative. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and. Iron Find The Atomic Mass.
From periodictable.me
Way to Find Atomic Mass of Elements with Examples Iron Find The Atomic Mass By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Identify the charge and relative mass. Iron Find The Atomic Mass.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID4707302 Iron Find The Atomic Mass How to calculate atomic mass. This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. The atomic mass or relative. The atomic mass for each element is given in atomic mass units or grams per mole of atoms. For a single atom, atomic mass. Iron Find The Atomic Mass.
From otissan.blogspot.com
Molar Mass Of Iron Calculate the molar mass of aluminum oxide (Al2O3 Iron Find The Atomic Mass For a single atom, atomic mass is the sum of the protons and neutrons. The atomic mass is the mass of an atom. By calculating an average of an element’s atomic masses, weighted by the natural abundance of each isotope, we obtain a weighted average mass called the atomic mass (also. This atomic mass calculator shows you how to find. Iron Find The Atomic Mass.
From syatillakmk.blogspot.com
SimplyChemistry C1 1.2RELATIVE ATOMIC MASS (R.A.M) Iron Find The Atomic Mass This atomic mass calculator shows you how to find the atomic mass of an atom using the atomic mass formula and explains the atomic mass definition. Atomic mass of iron is 55.845 u. How to calculate atomic mass. Determine the number of protons, neutrons, and electrons in an atom. Define atomic and mass numbers. Review the steps to find atomic. Iron Find The Atomic Mass.
From utedzz.blogspot.com
Periodic Table Iron Atomic Mass Periodic Table Timeline Iron Find The Atomic Mass The easiest way to find the atomic mass is to look it up on a periodic table. The atomic mass is the mass of an atom. Identify the charge and relative mass of subatomic particles. The atomic mass or relative. For a single atom, atomic mass is the sum of the protons and neutrons. Atomic mass of iron is 55.845. Iron Find The Atomic Mass.
From www.slideserve.com
PPT The mass of particles PowerPoint Presentation, free download ID Iron Find The Atomic Mass Define atomic and mass numbers. Identify the charge and relative mass of subatomic particles. For a single atom, atomic mass is the sum of the protons and neutrons. The atomic mass or relative. The easiest way to find the atomic mass is to look it up on a periodic table. Review the steps to find atomic mass. This atomic mass. Iron Find The Atomic Mass.