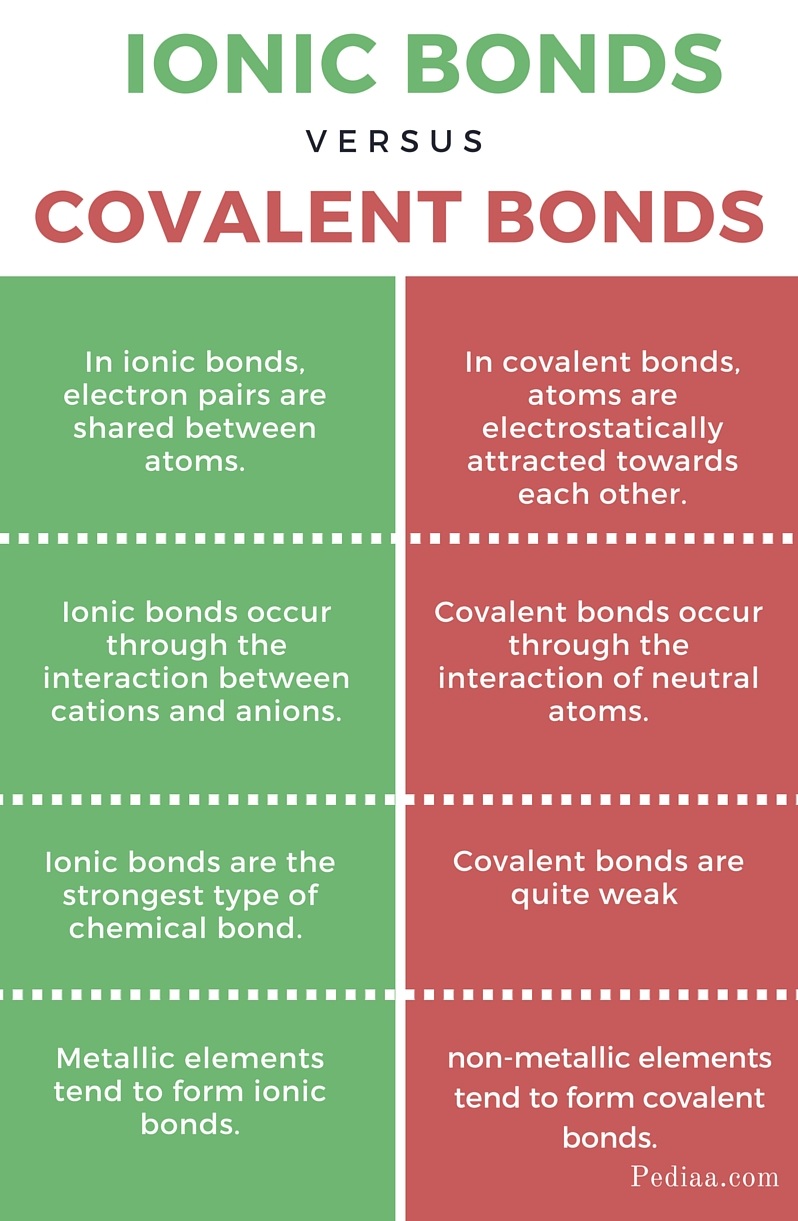
What Are Ionic Compounds And Covalent Compounds . Predict the type of compound formed from elements based on their location within the. For the most part, ionic compounds contain a metal bonded to a nonmetal. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. Define ionic and molecular (covalent) compounds. The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? Many ionic compounds are binary compounds formed by a metal and a nonmetal. Here is an explanation of the difference between ionic. Ionic compounds consist of atoms joined together by ionic bonds. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard.
from pediaa.com
A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Define ionic and molecular (covalent) compounds. For the most part, ionic compounds contain a metal bonded to a nonmetal. Many ionic compounds are binary compounds formed by a metal and a nonmetal. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. The first question we ask is if the compound is ionic or covalent? Ionic compounds consist of atoms joined together by ionic bonds. That is, does it have ionic bonds, or covalent bonds?
Difference Between Covalent and Ionic Bonds
What Are Ionic Compounds And Covalent Compounds The first question we ask is if the compound is ionic or covalent? That is, does it have ionic bonds, or covalent bonds? Many ionic compounds are binary compounds formed by a metal and a nonmetal. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Define ionic and molecular (covalent) compounds. Here is an explanation of the difference between ionic. The first question we ask is if the compound is ionic or covalent? A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. Ionic compounds consist of atoms joined together by ionic bonds. For the most part, ionic compounds contain a metal bonded to a nonmetal. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Predict the type of compound formed from elements based on their location within the.
From quizlet.com
Ionic and Covalent Bonds Diagram Quizlet What Are Ionic Compounds And Covalent Compounds That is, does it have ionic bonds, or covalent bonds? Ionic compounds consist of atoms joined together by ionic bonds. Many ionic compounds are binary compounds formed by a metal and a nonmetal. Define ionic and molecular (covalent) compounds. Predict the type of compound formed from elements based on their location within the. The first question we ask is if. What Are Ionic Compounds And Covalent Compounds.
From www.slideserve.com
PPT Ionic vs. Molecular Compounds PowerPoint Presentation, free What Are Ionic Compounds And Covalent Compounds The first question we ask is if the compound is ionic or covalent? Here is an explanation of the difference between ionic. For the most part, ionic compounds contain a metal bonded to a nonmetal. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic compounds consist of atoms. What Are Ionic Compounds And Covalent Compounds.
From biologydictionary.net
Ionic Bond Examples Biology Dictionary What Are Ionic Compounds And Covalent Compounds Predict the type of compound formed from elements based on their location within the. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. That is, does it have ionic bonds, or covalent bonds? A polar covalent bond is formed when. What Are Ionic Compounds And Covalent Compounds.
From infogram.com
Covalent Bonds .VS. Ionic Bonds Infogram What Are Ionic Compounds And Covalent Compounds The first question we ask is if the compound is ionic or covalent? For the most part, ionic compounds contain a metal bonded to a nonmetal. Many ionic compounds are binary compounds formed by a metal and a nonmetal. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic. What Are Ionic Compounds And Covalent Compounds.
From sciencenotes.org
Compounds With Both Ionic and Covalent Bonds What Are Ionic Compounds And Covalent Compounds Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic compounds consist of atoms joined together by ionic bonds. A. What Are Ionic Compounds And Covalent Compounds.
From www.slideserve.com
PPT Chemical Bonds (Ionic and Covalent Bonds) Part 1 PowerPoint What Are Ionic Compounds And Covalent Compounds Predict the type of compound formed from elements based on their location within the. That is, does it have ionic bonds, or covalent bonds? The first question we ask is if the compound is ionic or covalent? Ionic compounds consist of atoms joined together by ionic bonds. For the most part, ionic compounds contain a metal bonded to a nonmetal.. What Are Ionic Compounds And Covalent Compounds.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds What Are Ionic Compounds And Covalent Compounds The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. The first question we ask is if the compound is ionic or covalent? Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. That is, does it have ionic bonds, or covalent bonds? Ionic compounds. What Are Ionic Compounds And Covalent Compounds.
From blogs.glowscotland.org.uk
Review Comparing Ionic/ Covalent Compounds S3 Chemistry Consolidation What Are Ionic Compounds And Covalent Compounds Here is an explanation of the difference between ionic. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. For the most part, ionic compounds contain a metal bonded to a nonmetal. That is, does it have ionic bonds, or covalent bonds? Define ionic and molecular (covalent) compounds. Many ionic compounds are binary compounds formed. What Are Ionic Compounds And Covalent Compounds.
From sciencenotes.org
Naming Covalent Compounds Nomenclature Rules What Are Ionic Compounds And Covalent Compounds For the most part, ionic compounds contain a metal bonded to a nonmetal. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Predict the type of compound formed from elements based on their location within the.. What Are Ionic Compounds And Covalent Compounds.
From www.youtube.com
Properties of Ionic and Covalent Compounds YouTube What Are Ionic Compounds And Covalent Compounds The first question we ask is if the compound is ionic or covalent? A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Many ionic compounds are binary compounds formed by a. What Are Ionic Compounds And Covalent Compounds.
From www.teachoo.com
[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo What Are Ionic Compounds And Covalent Compounds That is, does it have ionic bonds, or covalent bonds? Here is an explanation of the difference between ionic. For the most part, ionic compounds contain a metal bonded to a nonmetal. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. The main difference between ionic and covalent bonds is how. What Are Ionic Compounds And Covalent Compounds.
From www.youtube.com
Ionic Compounds vs. Covalent Molecules. (Chemistry Ch. 5, Part 5) YouTube What Are Ionic Compounds And Covalent Compounds Predict the type of compound formed from elements based on their location within the. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Many ionic compounds are binary compounds formed by a metal and. What Are Ionic Compounds And Covalent Compounds.
From www.expii.com
Ionic Bond — Formation & Compounds Expii What Are Ionic Compounds And Covalent Compounds Here is an explanation of the difference between ionic. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Predict the type of compound formed from elements based on their location within the. Many ionic compounds are binary compounds formed by a metal and a nonmetal. The first question we ask is if the compound is. What Are Ionic Compounds And Covalent Compounds.
From coredifferences.com
15 Major Difference between Covalent and Ionic Bonds with Table Core What Are Ionic Compounds And Covalent Compounds Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. For the most part, ionic compounds contain a metal bonded to a nonmetal. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. Predict the type of compound formed from elements based on their location within the. Define. What Are Ionic Compounds And Covalent Compounds.
From www.masterorganicchemistry.com
Ionic and Covalent Bonding Master Organic Chemistry What Are Ionic Compounds And Covalent Compounds A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. For the most part, ionic compounds contain a metal bonded to a nonmetal. That is, does it have ionic bonds, or covalent bonds? Ionic compounds form crystals,. What Are Ionic Compounds And Covalent Compounds.
From www.yourdictionary.com
Main Differences Between Ionic and Covalent Bonds YourDictionary What Are Ionic Compounds And Covalent Compounds Predict the type of compound formed from elements based on their location within the. Many ionic compounds are binary compounds formed by a metal and a nonmetal. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. That is, does it have ionic bonds, or covalent bonds? For the most. What Are Ionic Compounds And Covalent Compounds.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities What Are Ionic Compounds And Covalent Compounds Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Predict the type of compound formed from elements based on their location within the. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. That is, does it have ionic bonds, or covalent bonds? Many ionic compounds. What Are Ionic Compounds And Covalent Compounds.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica What Are Ionic Compounds And Covalent Compounds That is, does it have ionic bonds, or covalent bonds? Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Ionic compounds consist of atoms joined together by ionic bonds. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Define ionic and molecular (covalent) compounds. Predict the type of compound. What Are Ionic Compounds And Covalent Compounds.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica What Are Ionic Compounds And Covalent Compounds Ionic compounds consist of atoms joined together by ionic bonds. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. The first question we ask is if the compound is ionic or covalent? Predict the type of compound formed from elements based on their location within the. The main difference between ionic and covalent bonds is. What Are Ionic Compounds And Covalent Compounds.
From giovannigokesalas.blogspot.com
Difference Between Ionic and Covalent Bonds What Are Ionic Compounds And Covalent Compounds The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. The first question we ask is if the compound is ionic or covalent? Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete. What Are Ionic Compounds And Covalent Compounds.
From utedzz.blogspot.com
Periodic Table Ions List Periodic Table Timeline What Are Ionic Compounds And Covalent Compounds The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. The first question we ask is if the compound is ionic or covalent? Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Ionic compounds form crystals, typically have high melting and boiling points, are. What Are Ionic Compounds And Covalent Compounds.
From pediaa.com
Difference Between Covalent and Ionic Bonds What Are Ionic Compounds And Covalent Compounds Ionic compounds consist of atoms joined together by ionic bonds. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. That is, does it have ionic bonds, or covalent bonds? Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Define ionic and molecular (covalent) compounds. Many ionic compounds are binary. What Are Ionic Compounds And Covalent Compounds.
From www.youtube.com
Ionic and Covalent Bonding Chemistry YouTube What Are Ionic Compounds And Covalent Compounds Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Here is an explanation of the difference between ionic. A polar. What Are Ionic Compounds And Covalent Compounds.
From www.slideserve.com
PPT The Structure of Matter PowerPoint Presentation, free download What Are Ionic Compounds And Covalent Compounds Define ionic and molecular (covalent) compounds. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Predict the type of compound. What Are Ionic Compounds And Covalent Compounds.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities What Are Ionic Compounds And Covalent Compounds Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. That is, does it have ionic bonds, or covalent bonds? Ionic compounds consist of atoms joined together by ionic bonds. Many ionic compounds are binary compounds formed by a metal and a nonmetal. Define ionic and molecular (covalent) compounds. Describe ionic compounds as an extended three. What Are Ionic Compounds And Covalent Compounds.
From sciencenotes.org
Ionic vs Covalent Bonds What Are Ionic Compounds And Covalent Compounds The first question we ask is if the compound is ionic or covalent? For the most part, ionic compounds contain a metal bonded to a nonmetal. Here is an explanation of the difference between ionic. Define ionic and molecular (covalent) compounds. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Ionic compounds consist of. What Are Ionic Compounds And Covalent Compounds.
From sciencenotes.org
Covalent Compounds Examples and Properties What Are Ionic Compounds And Covalent Compounds Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Here is an explanation of the difference between ionic. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. A polar. What Are Ionic Compounds And Covalent Compounds.
From www.compoundworksheets.com
Identifying IONIC AND COVALENT COMPOUNDS Lewis Dot Diagrams Worksheet What Are Ionic Compounds And Covalent Compounds For the most part, ionic compounds contain a metal bonded to a nonmetal. Define ionic and molecular (covalent) compounds. Here is an explanation of the difference between ionic. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules in. Ionic compounds consist of atoms joined together by ionic bonds. Predict the type of compound formed from. What Are Ionic Compounds And Covalent Compounds.
From www.slideserve.com
PPT Covalent and Ionic Bonds PowerPoint Presentation, free download What Are Ionic Compounds And Covalent Compounds Here is an explanation of the difference between ionic. Predict the type of compound formed from elements based on their location within the. Many ionic compounds are binary compounds formed by a metal and a nonmetal. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. A polar covalent bond. What Are Ionic Compounds And Covalent Compounds.
From en.wikipedia.org
Ionic bonding Wikipedia What Are Ionic Compounds And Covalent Compounds The first question we ask is if the compound is ionic or covalent? Many ionic compounds are binary compounds formed by a metal and a nonmetal. That is, does it have ionic bonds, or covalent bonds? Ionic compounds consist of atoms joined together by ionic bonds. Describe ionic compounds as an extended three dimensional array, lattice structures, versus discrete molecules. What Are Ionic Compounds And Covalent Compounds.
From cartoondealer.com
How Ionic Compounds Are Formed RoyaltyFree Stock Photography What Are Ionic Compounds And Covalent Compounds Many ionic compounds are binary compounds formed by a metal and a nonmetal. Here is an explanation of the difference between ionic. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. The first question we ask is if the compound is ionic or covalent? Ionic compounds consist of atoms joined together by ionic bonds. The. What Are Ionic Compounds And Covalent Compounds.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures What Are Ionic Compounds And Covalent Compounds A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Define ionic and molecular (covalent) compounds. The first question we ask is if the compound is ionic or covalent? Describe ionic compounds as an extended three dimensional array,. What Are Ionic Compounds And Covalent Compounds.
From www.differencebetween.com
Difference Between Ionic and Covalent Compounds Compare the What Are Ionic Compounds And Covalent Compounds That is, does it have ionic bonds, or covalent bonds? Ionic compounds consist of atoms joined together by ionic bonds. Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. Here is an explanation of the difference between ionic. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms. What Are Ionic Compounds And Covalent Compounds.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart What Are Ionic Compounds And Covalent Compounds Ionic compounds form crystals, typically have high melting and boiling points, are usually hard. A polar covalent bond is formed when the nonmetals that share pairs of electrons have a greater electronegativity. Here is an explanation of the difference between ionic. Predict the type of compound formed from elements based on their location within the. The main difference between ionic. What Are Ionic Compounds And Covalent Compounds.
From sciencenotes.org
Naming Covalent Compounds Nomenclature Rules What Are Ionic Compounds And Covalent Compounds For the most part, ionic compounds contain a metal bonded to a nonmetal. Many ionic compounds are binary compounds formed by a metal and a nonmetal. Ionic compounds consist of atoms joined together by ionic bonds. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. The first question we. What Are Ionic Compounds And Covalent Compounds.