Dlt 3+3 Design . Select a target dlt rate, typically 30% or 25%, and. For the 3 + 3. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). design of 3+3 phase i clinical trials. predefine dose levels for escalation as if for a “3 + 3“ design. The traditional 3+3 design was originally introduced in. the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. in the “3+3+3” design, a third cohort of three patients is added if two of.
from 101blockchains.com
Select a target dlt rate, typically 30% or 25%, and. design of 3+3 phase i clinical trials. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. predefine dose levels for escalation as if for a “3 + 3“ design. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. The traditional 3+3 design was originally introduced in. in the “3+3+3” design, a third cohort of three patients is added if two of. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). For the 3 + 3.
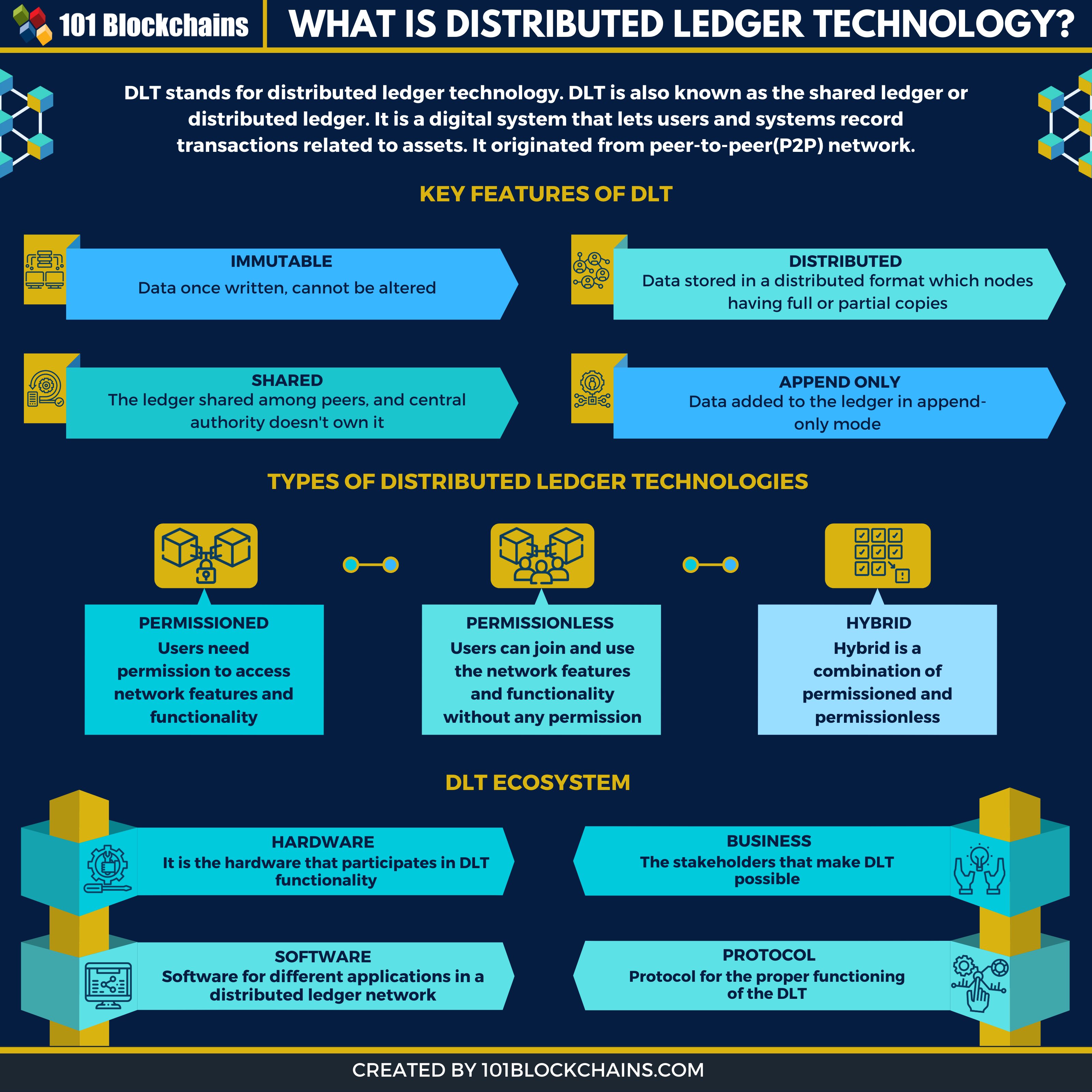
What is DLT (Distributed Ledger Technology) ? 101 Blockchains
Dlt 3+3 Design in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. Select a target dlt rate, typically 30% or 25%, and. design of 3+3 phase i clinical trials. in the “3+3+3” design, a third cohort of three patients is added if two of. predefine dose levels for escalation as if for a “3 + 3“ design. The traditional 3+3 design was originally introduced in. For the 3 + 3. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate.
From ascopubs.org
Moving Beyond 3+3 The Future of Clinical Trial Design American Dlt 3+3 Design in the “3+3+3” design, a third cohort of three patients is added if two of. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate.. Dlt 3+3 Design.
From www.slideserve.com
PPT ONE LUNG VENTILATION (OLV)SEPARATION Why & How ? PowerPoint Dlt 3+3 Design the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. in the “3+3+3” design, a third cohort of three patients is added if two of. For the 3 + 3. Select a target dlt rate, typically 30% or 25%, and. The traditional 3+3 design was originally introduced in.. Dlt 3+3 Design.
From www.researchgate.net
Healthcare SSI Architecture (1) Patient requests a credential (2 Dlt 3+3 Design the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. design of 3+3 phase i clinical trials. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. in phase i oncology trials, the primary goal is to assess dose limiting toxicities. Dlt 3+3 Design.
From dlthub.com
How dlt works dlt Docs Dlt 3+3 Design Select a target dlt rate, typically 30% or 25%, and. For the 3 + 3. design of 3+3 phase i clinical trials. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). The traditional 3+3 design was originally introduced in. predefine dose levels for escalation. Dlt 3+3 Design.
From 101blockchains.com
What is DLT (Distributed Ledger Technology) ? 101 Blockchains Dlt 3+3 Design in the “3+3+3” design, a third cohort of three patients is added if two of. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. . Dlt 3+3 Design.
From aikchinhin.sg
DLT CRIMPING TOOL DLT3S Model CT2DLT3S Dlt 3+3 Design in the “3+3+3” design, a third cohort of three patients is added if two of. The traditional 3+3 design was originally introduced in. For the 3 + 3. design of 3+3 phase i clinical trials. predefine dose levels for escalation as if for a “3 + 3“ design. 3+3 design is the most commonly used design. Dlt 3+3 Design.
From www.researchgate.net
A) Schematic of the standard 3 + 3 design. (B) Schematic of the Dlt 3+3 Design the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). in the “3+3+3” design, a third cohort of three patients is added if two of.. Dlt 3+3 Design.
From www.guardianglass.com
Massachusetts Department of Transportation District 3 Administration Dlt 3+3 Design The traditional 3+3 design was originally introduced in. design of 3+3 phase i clinical trials. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. Select a target dlt rate, typically 30% or 25%, and. in phase i oncology trials, the primary goal is to assess dose. Dlt 3+3 Design.
From www.wuxiroad.com
DLT3系列沥青脱桶装置 无锡路德筑路机械科技有限公司 Dlt 3+3 Design design of 3+3 phase i clinical trials. For the 3 + 3. the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. The traditional 3+3 design was originally introduced in. . Dlt 3+3 Design.
From www.slideserve.com
PPT Designs for Oncology MTD Finding PowerPoint Presentation, free Dlt 3+3 Design Select a target dlt rate, typically 30% or 25%, and. in the “3+3+3” design, a third cohort of three patients is added if two of. design of 3+3 phase i clinical trials. For the 3 + 3. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated. Dlt 3+3 Design.
From constructandcommission.com
DATA CENTER TIERS 1, 2, 3 & 4 Explained With Downloads Dlt 3+3 Design 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. Select a target dlt rate, typically 30% or 25%, and. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. the 3+3 design defines the mtd as the highest dose with no. Dlt 3+3 Design.
From www.bhinneka.com
√ Harga DELTA DLT 33 Series DLT SRV 330100 Terbaru Bhinneka Dlt 3+3 Design the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. design of 3+3 phase i clinical trials. The traditional 3+3 design was originally introduced in. in the “3+3+3” design, a third cohort of three patients is added if two of. in phase i oncology trials, the. Dlt 3+3 Design.
From www.researchgate.net
The 3+3 dose escalation study design. Download Scientific Diagram Dlt 3+3 Design 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. predefine dose levels for escalation as if for a “3 + 3“ design. design of 3+3 phase i clinical trials. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose. Dlt 3+3 Design.
From stock.adobe.com
DLT letter logo. DLT best white background vector image. DLT Monogram Dlt 3+3 Design in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). predefine dose levels for escalation as if for a “3 + 3“ design. For the 3 + 3. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. The traditional. Dlt 3+3 Design.
From www.researchgate.net
Displaced leftturn (DLT) layout (partial DLT with two crossovers Dlt 3+3 Design predefine dose levels for escalation as if for a “3 + 3“ design. design of 3+3 phase i clinical trials. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. in the “3+3+3” design, a third cohort of three patients is added if two of. The traditional 3+3 design was originally. Dlt 3+3 Design.
From www.researchgate.net
Study flow chart. DLT, doselimiting toxicity. Download Scientific Dlt 3+3 Design in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). For the 3 + 3. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. predefine dose levels for escalation as if for a “3. Dlt 3+3 Design.
From www.bhinneka.com
DELTA DLT 33 Series DLT SRV HI 330250 Dlt 3+3 Design Select a target dlt rate, typically 30% or 25%, and. predefine dose levels for escalation as if for a “3 + 3“ design. design of 3+3 phase i clinical trials. For the 3 + 3. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd).. Dlt 3+3 Design.
From insideevs.com
New Tesla Model 3 Debuts Chiseled Design, 421Mile Estimated Range Dlt 3+3 Design For the 3 + 3. design of 3+3 phase i clinical trials. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). predefine dose levels for escalation as if for a “3 + 3“ design. Select a target dlt rate, typically 30% or 25%, and.. Dlt 3+3 Design.
From zhuanlan.zhihu.com
AUTOSAR DLT (Diagnostic Log and Trace) 知乎 Dlt 3+3 Design in the “3+3+3” design, a third cohort of three patients is added if two of. For the 3 + 3. The traditional 3+3 design was originally introduced in. design of 3+3 phase i clinical trials. the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. Select. Dlt 3+3 Design.
From www.researchgate.net
Escalation scheme for 3+3 design with dose deescalation (adapted from Dlt 3+3 Design For the 3 + 3. Select a target dlt rate, typically 30% or 25%, and. predefine dose levels for escalation as if for a “3 + 3“ design. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. the 3+3 design defines the mtd as the highest dose with no more than. Dlt 3+3 Design.
From www.researchgate.net
Schematic display of one version of the 3 + 3 design Download Dlt 3+3 Design in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. predefine dose levels for escalation as if for a “3 + 3“ design. . Dlt 3+3 Design.
From kbase.zetaris.com
Azure Databricks Dlt 3+3 Design in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. predefine dose levels for escalation as if for a “3 + 3“ design. . Dlt 3+3 Design.
From www.researchgate.net
Threeplusthree dose escalation design. DLT, doselimiting toxicity Dlt 3+3 Design the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. For the 3 + 3. predefine dose levels for escalation as if for a “3 + 3“ design. The traditional 3+3 design was originally introduced in. the 3+3 design defines the mtd as the highest dose with. Dlt 3+3 Design.
From www.sec.gov
Slide 28 Dlt 3+3 Design For the 3 + 3. in the “3+3+3” design, a third cohort of three patients is added if two of. predefine dose levels for escalation as if for a “3 + 3“ design. design of 3+3 phase i clinical trials. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. . Dlt 3+3 Design.
From dlthub.com
dlt AI Assistant provides answers you need! dlt Docs Dlt 3+3 Design predefine dose levels for escalation as if for a “3 + 3“ design. design of 3+3 phase i clinical trials. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). For the 3 + 3. 3+3 design is the most commonly used design in. Dlt 3+3 Design.
From blog.csdn.net
AUTOSAR DiagnosticLogAndTrace DLT(三) 消息的发送、DLT命令的发送与接收_autosar dltCSDN博客 Dlt 3+3 Design design of 3+3 phase i clinical trials. predefine dose levels for escalation as if for a “3 + 3“ design. The traditional 3+3 design was originally introduced in. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. 3+3 design is the most commonly used design. Dlt 3+3 Design.
From nampharma.com
DLT3 Water Soluble Powder Nam Pharma Sdn. Bhd. Dlt 3+3 Design The traditional 3+3 design was originally introduced in. For the 3 + 3. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. Select a. Dlt 3+3 Design.
From github.com
GitHub souvikdatabricks/dltwithdebug A lightweight helper utility Dlt 3+3 Design The traditional 3+3 design was originally introduced in. predefine dose levels for escalation as if for a “3 + 3“ design. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. Select. Dlt 3+3 Design.
From www.slideserve.com
PPT Novel Targeted Drugs and Their Introduction to the Clinic Dlt 3+3 Design Select a target dlt rate, typically 30% or 25%, and. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. predefine dose levels for escalation as if for a “3 + 3“ design. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt. Dlt 3+3 Design.
From design1systems.com
StepbyStep Guide Craftsman DLT 3000 Deck Belt Diagram Explained Dlt 3+3 Design For the 3 + 3. predefine dose levels for escalation as if for a “3 + 3“ design. design of 3+3 phase i clinical trials. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). the acceptable proportion of patients that defines the mtd. Dlt 3+3 Design.
From mungfali.com
Dowel Laminated Timber Dlt 3+3 Design 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. The traditional 3+3 design was originally introduced in. For the 3 + 3. in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). the acceptable proportion of patients that defines. Dlt 3+3 Design.
From onbiostatistics.blogspot.com
On Biostatistics and Clinical Trials Phase I Dose Escalation Study Dlt 3+3 Design in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. For the 3 + 3. design of 3+3 phase i clinical trials. 3+3. Dlt 3+3 Design.
From www.celinehh.com
Phase I Clinical Trials — Celine Halioua Dlt 3+3 Design in phase i oncology trials, the primary goal is to assess dose limiting toxicities (dlt) and estimate the maximum tolerated dose (mtd). Select a target dlt rate, typically 30% or 25%, and. predefine dose levels for escalation as if for a “3 + 3“ design. design of 3+3 phase i clinical trials. the 3+3 design defines. Dlt 3+3 Design.
From www.slideserve.com
PPT Novel Targeted Drugs and Their Introduction to the Clinic Dlt 3+3 Design The traditional 3+3 design was originally introduced in. Select a target dlt rate, typically 30% or 25%, and. 3+3 design is the most commonly used design in conducting phase i cancer clinical trials. predefine dose levels for escalation as if for a “3 + 3“ design. For the 3 + 3. in phase i oncology trials, the. Dlt 3+3 Design.
From autosartutorials.com
Diagnostic Log and Trace in AUTOSAR Dlt module in AUTOSAR Dlt 3+3 Design Select a target dlt rate, typically 30% or 25%, and. For the 3 + 3. the 3+3 design defines the mtd as the highest dose with no more than 1 out of 6 patients experiencing dlt. the acceptable proportion of patients that defines the mtd for the study is referred to as the target dlt rate. predefine. Dlt 3+3 Design.