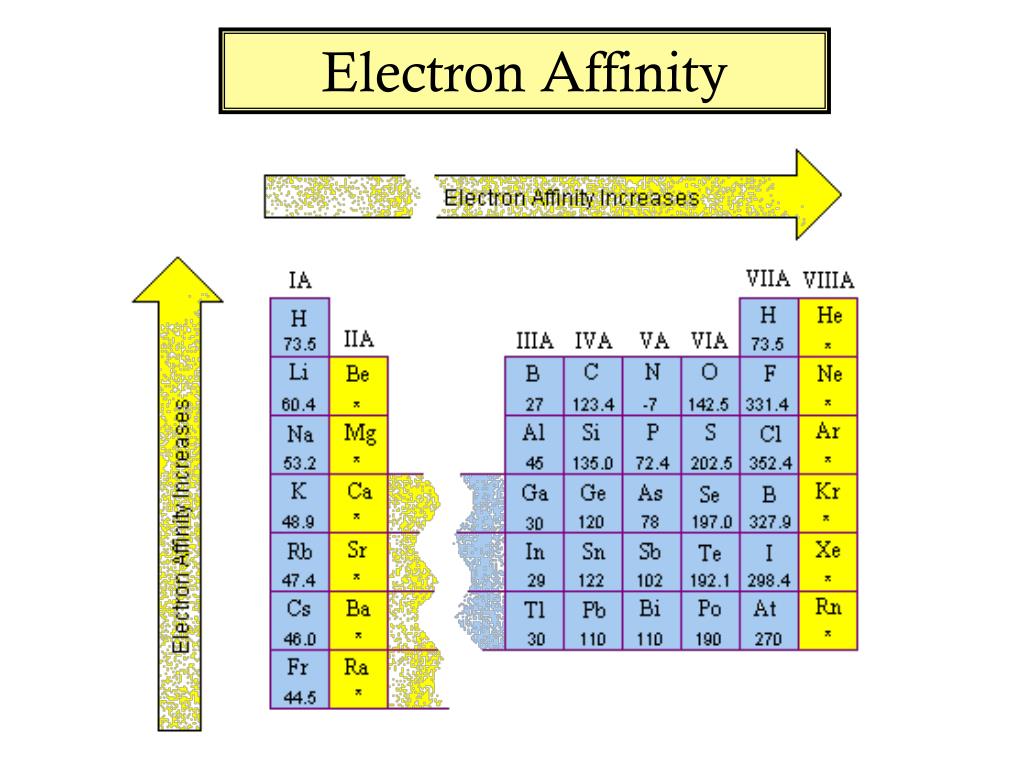
What Is Electron Affinity In Periodic Table . Basically, energy is required in order. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors electron affinity is a measure of the energy released when an extra electron is added to an atom. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. Electron affinities are measured in the gaseous. what is electron affinity. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic.
from www.slideserve.com
what is electron affinity. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors Basically, energy is required in order. Electron affinities are measured in the gaseous. electron affinity is a measure of the energy released when an extra electron is added to an atom. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an.
PPT Chapter 4 The Periodic Table PowerPoint Presentation, free
What Is Electron Affinity In Periodic Table electron affinity is a measure of the energy released when an extra electron is added to an atom. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. Electron affinities are measured in the gaseous. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. what is electron affinity. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. Basically, energy is required in order. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. electron affinity is a measure of the energy released when an extra electron is added to an atom.
From unilasopa610.weebly.com
What is electron affinity unilasopa What Is Electron Affinity In Periodic Table what is electron affinity. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Electron affinities are measured in the gaseous. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors Basically, energy is required in. What Is Electron Affinity In Periodic Table.
From www.breakingatom.com
Electron Affinity of The Elements What Is Electron Affinity In Periodic Table electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. electron affinity is a measure of the energy released when an extra electron is added to an atom. Electron affinities are measured in the gaseous. electron affinity for most atoms on the periodic table, except the noble. What Is Electron Affinity In Periodic Table.
From periodictableguide.com
Electron Affinity Chart (Labeled Periodic table + List) What Is Electron Affinity In Periodic Table electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors Basically, energy is required in order. The electron affinity is defined as the amount of energy released per mole when an. What Is Electron Affinity In Periodic Table.
From www.youtube.com
What is Electron Affinity ? Its Factors and Trends in periodic table What Is Electron Affinity In Periodic Table electron affinity is a measure of the energy released when an extra electron is added to an atom. Basically, energy is required in order. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. The electron affinity is defined as the amount of energy released per mole when. What Is Electron Affinity In Periodic Table.
From ar.inspiredpencil.com
Periodic Table With Electron Affinity What Is Electron Affinity In Periodic Table what is electron affinity. Basically, energy is required in order. Electron affinities are measured in the gaseous. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. . What Is Electron Affinity In Periodic Table.
From chemwiki.ucdavis.edu
Electronic Configurations Chemwiki What Is Electron Affinity In Periodic Table The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in. What Is Electron Affinity In Periodic Table.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps What Is Electron Affinity In Periodic Table The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase). What Is Electron Affinity In Periodic Table.
From atonce.com
50 Key Periodic Trends in Electron Affinity 2024 Guide What Is Electron Affinity In Periodic Table The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. What Is Electron Affinity In Periodic Table.
From www.sliderbase.com
Periodic Behavior Presentation Chemistry What Is Electron Affinity In Periodic Table electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Basically, energy is required in order. what is electron affinity. learn all. What Is Electron Affinity In Periodic Table.
From commons.wikimedia.org
Original file (SVG file, nominally 851 × 520 pixels, file size 91 KB) What Is Electron Affinity In Periodic Table electron affinity is a measure of the energy released when an extra electron is added to an atom. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in. What Is Electron Affinity In Periodic Table.
From ppt-online.org
The Periodic Table презентация онлайн What Is Electron Affinity In Periodic Table Electron affinities are measured in the gaseous. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 119 rows electron affinity is the amount of energy change (δe) that occurs when an. What Is Electron Affinity In Periodic Table.
From www.slideserve.com
PPT Chapter 4 The Periodic Table PowerPoint Presentation, free What Is Electron Affinity In Periodic Table 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. electron affinity is a measure of the energy released when an extra electron. What Is Electron Affinity In Periodic Table.
From pediabay.com
Electron Affinity Chart of Elements (With Periodic Table) Pediabay What Is Electron Affinity In Periodic Table The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. what is electron affinity. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors Basically, energy is required in order. electron affinity for most atoms on the periodic. What Is Electron Affinity In Periodic Table.
From ar.inspiredpencil.com
Electron Affinity Periodic Table What Is Electron Affinity In Periodic Table Electron affinities are measured in the gaseous. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. electron affinity is defined as the change in energy (in kj/mole). What Is Electron Affinity In Periodic Table.
From pubchem.ncbi.nlm.nih.gov
Electron Affinity Periodic Table of Elements PubChem What Is Electron Affinity In Periodic Table 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. Basically, energy is required in order. electron affinity is a measure of. What Is Electron Affinity In Periodic Table.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table What Is Electron Affinity In Periodic Table what is electron affinity. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors The electron affinity is defined as the amount of energy released per mole when an electron. What Is Electron Affinity In Periodic Table.
From knordslearning.com
Electron Affinity Trend in Periodic Table (Explained) What Is Electron Affinity In Periodic Table what is electron affinity. Basically, energy is required in order. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. learn all about electron affinity, including it's definition, periodic table trends. What Is Electron Affinity In Periodic Table.
From www.pinterest.com
Electron Affinity Electron affinity, Periodic table, Chemistry for kids What Is Electron Affinity In Periodic Table electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Electron affinities are measured in the gaseous. what is electron affinity. learn. What Is Electron Affinity In Periodic Table.
From www.studypool.com
SOLUTION Periodic table of elements w electron affinity pubchem What Is Electron Affinity In Periodic Table Electron affinities are measured in the gaseous. electron affinity is a measure of the energy released when an extra electron is added to an atom. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. what is electron affinity. 119 rows electron affinity is the amount of energy change (δe) that. What Is Electron Affinity In Periodic Table.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2834450 What Is Electron Affinity In Periodic Table Electron affinities are measured in the gaseous. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors electron affinity is a measure of the energy released when an extra electron is added to an atom. 119 rows electron affinity is the amount of energy change (δe) that occurs when an. What Is Electron Affinity In Periodic Table.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Is Electron Affinity In Periodic Table The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. what is electron affinity. Electron affinities are measured in the gaseous. electron affinity is a measure of the energy released when an extra electron is added to an atom. learn all about electron affinity, including. What Is Electron Affinity In Periodic Table.
From wisc.pb.unizin.org
D4.4 Periodic Variation in Electron Affinities Chem 109 Fall 2023 What Is Electron Affinity In Periodic Table what is electron affinity. Electron affinities are measured in the gaseous. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. electron affinity is a. What Is Electron Affinity In Periodic Table.
From www.youtube.com
Electron Affinity Part2 Chemistry General Concepts YouTube What Is Electron Affinity In Periodic Table electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. what is electron affinity. electron affinity is a measure of the energy released when an. What Is Electron Affinity In Periodic Table.
From www.shutterstock.com
Electron Affinity Values Periodic Table vetor stock (livre de direitos What Is Electron Affinity In Periodic Table electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. Basically, energy is required in order. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase). What Is Electron Affinity In Periodic Table.
From ar.inspiredpencil.com
Periodic Table With Electron Affinity What Is Electron Affinity In Periodic Table Electron affinities are measured in the gaseous. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. what is electron affinity. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors electron affinity is a measure of the. What Is Electron Affinity In Periodic Table.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table What Is Electron Affinity In Periodic Table what is electron affinity. Electron affinities are measured in the gaseous. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors electron affinity is a measure of the energy released when an extra electron is added to an atom. Basically, energy is required in order. The electron affinity is defined. What Is Electron Affinity In Periodic Table.
From www.breakingatom.com
Electron Affinity of The Elements What Is Electron Affinity In Periodic Table what is electron affinity. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. Electron affinities are measured in the gaseous. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. Basically, energy is required in order. The electron affinity is defined. What Is Electron Affinity In Periodic Table.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint What Is Electron Affinity In Periodic Table electron affinity is a measure of the energy released when an extra electron is added to an atom. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. what is electron affinity. Electron affinities are measured in the gaseous. electron affinity is defined as the change in energy (in kj/mole) of. What Is Electron Affinity In Periodic Table.
From chemistnotes.com
Electron Affinity Definition, Trends, and Equation Chemistry Notes What Is Electron Affinity In Periodic Table electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell. What Is Electron Affinity In Periodic Table.
From sciencenotes.org
Periodicity Definition in Chemistry What Is Electron Affinity In Periodic Table what is electron affinity. Basically, energy is required in order. electron affinity is a measure of the energy released when an extra electron is added to an atom. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. electron affinity for most atoms on the. What Is Electron Affinity In Periodic Table.
From www.researchgate.net
1 Periodic Table of Electron Affinities. All reported values are for What Is Electron Affinity In Periodic Table The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in. What Is Electron Affinity In Periodic Table.
From ar.inspiredpencil.com
Periodic Table With Electron Affinity What Is Electron Affinity In Periodic Table electron affinity is a measure of the energy released when an extra electron is added to an atom. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in. What Is Electron Affinity In Periodic Table.
From periodictable-1.blogspot.com
affinity periodic electron table highest Periodic affinities electron What Is Electron Affinity In Periodic Table electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. Basically, energy is required in order. electron affinity is a measure of the energy released when an extra electron is added to. What Is Electron Affinity In Periodic Table.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) What Is Electron Affinity In Periodic Table electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. Basically, energy is required in order. electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when. learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial. What Is Electron Affinity In Periodic Table.
From www.pearson.com
The periodic table with electron affinity values is shown below What Is Electron Affinity In Periodic Table learn all about electron affinity, including it's definition, periodic table trends & charts, exceptions and influencial factors electron affinity is a measure of the energy released when an extra electron is added to an atom. electron affinity for most atoms on the periodic table, except the noble gases, is exothermic. Basically, energy is required in order. The. What Is Electron Affinity In Periodic Table.