Aluminum Chloride Cation And Anion . Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. Name monoatomic anions and cations. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. It is composed of the metal cation aluminum and the nonmetal anion chloride. the aluminum chloride formula is {eq}alcl_3 {/eq}; predict the charge of monatomic main group elements based on their group number.
from www.lpdlabservices.co.uk
a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. predict the charge of monatomic main group elements based on their group number. the aluminum chloride formula is {eq}alcl_3 {/eq}; aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. Name monoatomic anions and cations. It is composed of the metal cation aluminum and the nonmetal anion chloride.
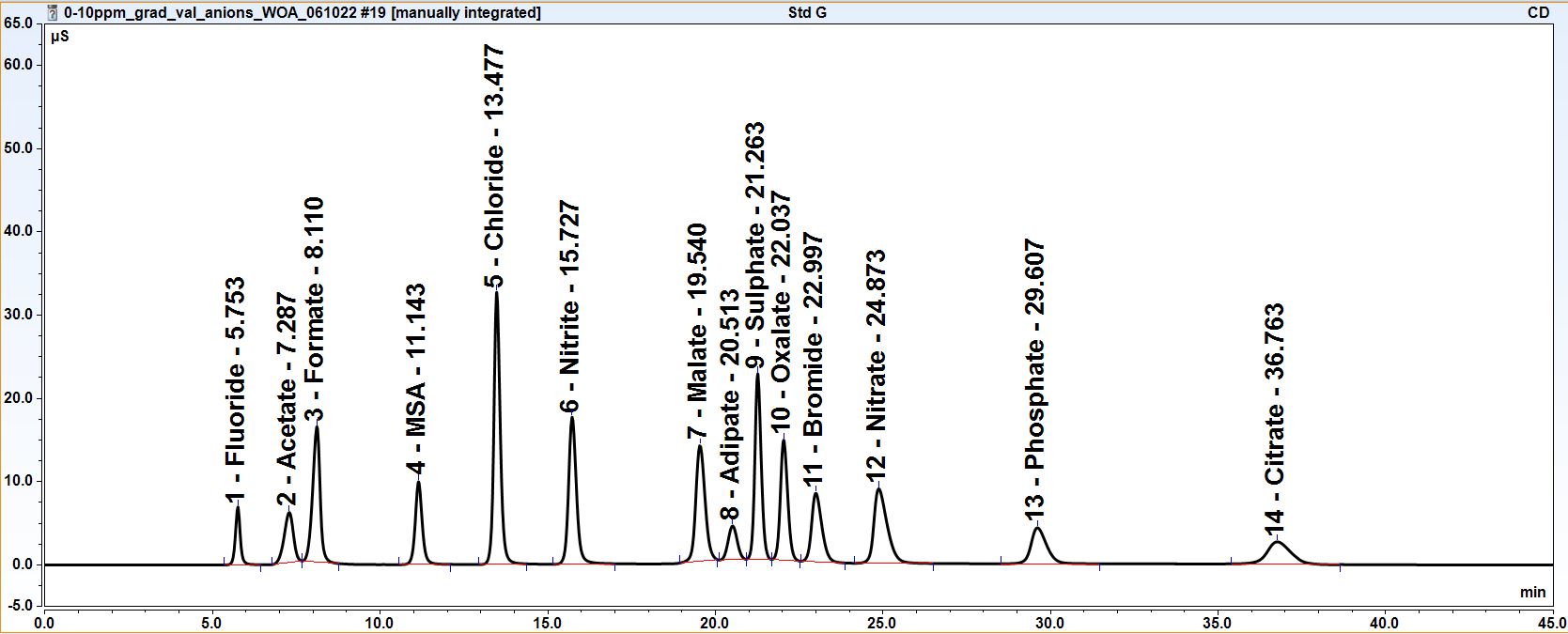
Anion and Cation Chromatography
Aluminum Chloride Cation And Anion the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. predict the charge of monatomic main group elements based on their group number. Name monoatomic anions and cations. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. the aluminum chloride formula is {eq}alcl_3 {/eq}; Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. It is composed of the metal cation aluminum and the nonmetal anion chloride.
From www.numerade.com
SOLVED For the substance shown, write the balanced dissolution Aluminum Chloride Cation And Anion aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. predict the charge of monatomic main group elements based on their group number. . Aluminum Chloride Cation And Anion.
From revivalportal.goodwood.com
Cations And Anions Chart Aluminum Chloride Cation And Anion predict the charge of monatomic main group elements based on their group number. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. It is composed of the metal cation aluminum and the nonmetal anion chloride. a cation (a positive ion) forms when a neutral atom loses one. Aluminum Chloride Cation And Anion.
From saylordotorg.github.io
Naming Ionic Compounds Aluminum Chloride Cation And Anion aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. Name monoatomic anions and cations. the aluminum chloride formula is {eq}alcl_3 {/eq}; Write formulas for ionic compounds using. Aluminum Chloride Cation And Anion.
From byjus.com
Explain cationic and anionic hydrolysis. Aqueous soln of aluminium Aluminum Chloride Cation And Anion Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. predict the charge of monatomic main group elements based on their group number. the aluminum chloride formula is {eq}alcl_3 {/eq}; Name. Aluminum Chloride Cation And Anion.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Aluminum Chloride Cation And Anion predict the charge of monatomic main group elements based on their group number. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. It is composed of the metal cation aluminum and the nonmetal anion chloride. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose. Aluminum Chloride Cation And Anion.
From www.numerade.com
SOLVED Prelaboratory Assignment 1. Give the formulas for the component Aluminum Chloride Cation And Anion the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. It is composed of the metal cation aluminum and the nonmetal anion chloride. Write formulas for ionic compounds using. Aluminum Chloride Cation And Anion.
From www.slideshare.net
Ch 8 ionic compounds Aluminum Chloride Cation And Anion the aluminum chloride formula is {eq}alcl_3 {/eq}; the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. Name monoatomic anions and cations. predict the charge of monatomic main group elements based on their group number. It is composed of the metal cation aluminum and the nonmetal anion. Aluminum Chloride Cation And Anion.
From slideplayer.com
Introduction Physical and Chemical Changes ppt download Aluminum Chloride Cation And Anion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of. Aluminum Chloride Cation And Anion.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Aluminum Chloride Cation And Anion the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. predict the charge of monatomic main group elements based on their group number. aluminium chloride is an ionic compound, as. Aluminum Chloride Cation And Anion.
From en.wikipedia.org
Ionic bonding Wikipedia Aluminum Chloride Cation And Anion the aluminum chloride formula is {eq}alcl_3 {/eq}; It is composed of the metal cation aluminum and the nonmetal anion chloride. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. predict the charge of monatomic main group elements based on their group number. Name monoatomic anions and. Aluminum Chloride Cation And Anion.
From chem.libretexts.org
4.3 Lewis Symbols and Structures Chemistry LibreTexts Aluminum Chloride Cation And Anion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. the aluminum chloride formula is {eq}alcl_3 {/eq}; aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. It is composed of the metal cation. Aluminum Chloride Cation And Anion.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Aluminum Chloride Cation And Anion the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. It is composed of the metal cation aluminum and the nonmetal anion chloride. Write formulas for ionic compounds using. Aluminum Chloride Cation And Anion.
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell Aluminum Chloride Cation And Anion It is composed of the metal cation aluminum and the nonmetal anion chloride. Name monoatomic anions and cations. predict the charge of monatomic main group elements based on their group number. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. the lighter. Aluminum Chloride Cation And Anion.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Aluminum Chloride Cation And Anion aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. predict the charge of monatomic main group elements based on their group number. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. Name monoatomic anions and cations.. Aluminum Chloride Cation And Anion.
From slideplayer.com
Naming Cations Cations When a metal loses it’s valence electron(s) it Aluminum Chloride Cation And Anion predict the charge of monatomic main group elements based on their group number. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Name monoatomic anions and. Aluminum Chloride Cation And Anion.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID6836286 Aluminum Chloride Cation And Anion aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. It is composed of the metal cation aluminum and the nonmetal anion chloride. Name monoatomic anions and cations. predict the charge of monatomic main group elements based on their group number. Write formulas for ionic compounds using monatomic and. Aluminum Chloride Cation And Anion.
From www.numerade.com
SOLVED Ionic Compounds Simple Metal + Simple NonMetal Name Cation Aluminum Chloride Cation And Anion Name monoatomic anions and cations. It is composed of the metal cation aluminum and the nonmetal anion chloride. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. the aluminum chloride formula is {eq}alcl_3 {/eq}; predict the charge of monatomic main group elements based on their group number. aluminium chloride. Aluminum Chloride Cation And Anion.
From slideplayer.com
Molecules, Compounds & Chemical Reactions ppt download Aluminum Chloride Cation And Anion Name monoatomic anions and cations. It is composed of the metal cation aluminum and the nonmetal anion chloride. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3. Aluminum Chloride Cation And Anion.
From slideplayer.com
Chemical Bonding. ppt download Aluminum Chloride Cation And Anion the aluminum chloride formula is {eq}alcl_3 {/eq}; the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. It is composed of the metal cation aluminum and the nonmetal anion chloride. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine,. Aluminum Chloride Cation And Anion.
From www.numerade.com
SOLVED Table 4 Ionic Compounds and their Names Formula of Cation and Aluminum Chloride Cation And Anion Name monoatomic anions and cations. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. predict the charge of monatomic main group elements based on their group number. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge. Aluminum Chloride Cation And Anion.
From www.coursehero.com
[Solved] what are aluminum acetate cation and anion and formula? what Aluminum Chloride Cation And Anion Name monoatomic anions and cations. predict the charge of monatomic main group elements based on their group number. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. the aluminum chloride formula is {eq}alcl_3 {/eq}; aluminium chloride is an ionic compound, as it is made up of aluminium, a cation,. Aluminum Chloride Cation And Anion.
From chemnotcheem.com
How to find the cation and anion in a compound? O Level Chemistry Aluminum Chloride Cation And Anion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle. Aluminum Chloride Cation And Anion.
From www.lpdlabservices.co.uk
Anion and Cation Chromatography Aluminum Chloride Cation And Anion aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion. Aluminum Chloride Cation And Anion.
From slideplayer.com
Nomenclature. ppt download Aluminum Chloride Cation And Anion Name monoatomic anions and cations. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. It is composed of the metal cation aluminum and the nonmetal anion chloride. predict the charge of monatomic main group elements based on their group number. aluminium chloride. Aluminum Chloride Cation And Anion.
From slideplayer.com
Chapter 7 Compounds and Their Bonds ppt download Aluminum Chloride Cation And Anion the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. the aluminum chloride formula is {eq}alcl_3 {/eq}; a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Name monoatomic anions and cations. . Aluminum Chloride Cation And Anion.
From www.youtube.com
How to Draw the Lewis Structure for AlCl3 Aluminum Chloride YouTube Aluminum Chloride Cation And Anion It is composed of the metal cation aluminum and the nonmetal anion chloride. the aluminum chloride formula is {eq}alcl_3 {/eq}; Name monoatomic anions and cations. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. aluminium chloride is an ionic compound, as it is made up of. Aluminum Chloride Cation And Anion.
From www.slideserve.com
PPT 1 Name the ions formed by these elements and classify them as Aluminum Chloride Cation And Anion It is composed of the metal cation aluminum and the nonmetal anion chloride. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Name monoatomic anions and cations. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative.. Aluminum Chloride Cation And Anion.
From www.slideserve.com
PPT 1 Name the ions formed by these elements and classify them as Aluminum Chloride Cation And Anion aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. the aluminum chloride formula is {eq}alcl_3 {/eq}; Write formulas for ionic compounds using monatomic and polyatomic ions by. Aluminum Chloride Cation And Anion.
From www.slideserve.com
PPT Chemical Nomenclature PowerPoint Presentation, free download ID Aluminum Chloride Cation And Anion aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. It is composed of the metal cation aluminum and the nonmetal anion chloride. Name monoatomic anions and cations. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an. Aluminum Chloride Cation And Anion.
From slideplayer.com
Chemical Nomenclature ppt download Aluminum Chloride Cation And Anion Name monoatomic anions and cations. a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. the aluminum chloride formula is {eq}alcl_3 {/eq}; the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. It. Aluminum Chloride Cation And Anion.
From slideplayer.com
Naming Compounds ppt download Aluminum Chloride Cation And Anion predict the charge of monatomic main group elements based on their group number. Name monoatomic anions and cations. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion. It is composed of. Aluminum Chloride Cation And Anion.
From www.numerade.com
SOLVEDClassify each of the following as a monoatomic cation Aluminum Chloride Cation And Anion the aluminum chloride formula is {eq}alcl_3 {/eq}; Name monoatomic anions and cations. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. predict the charge of monatomic main group elements based on their group number. the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose. Aluminum Chloride Cation And Anion.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps Aluminum Chloride Cation And Anion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. It is composed of the metal cation aluminum and the nonmetal anion chloride. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. predict the charge of. Aluminum Chloride Cation And Anion.
From robot.ekstrabladet.dk
Cátions E ânions Tabela Aluminum Chloride Cation And Anion the lighter group 3a metals (aluminum, galium and indium), along with scandium and yttrium lose 3 electrons to form [+3]. predict the charge of monatomic main group elements based on their group number. Name monoatomic anions and cations. aluminium chloride is an ionic compound, as it is made up of aluminium, a cation, and chlorine, an anion.. Aluminum Chloride Cation And Anion.
From slideplayer.com
Chapter 2 Atoms, Molecules, and Ions ppt download Aluminum Chloride Cation And Anion a cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative. Name monoatomic anions and cations. the aluminum chloride formula is {eq}alcl_3 {/eq}; It is composed of the metal cation aluminum and the nonmetal anion chloride. the lighter group 3a metals (aluminum, galium and. Aluminum Chloride Cation And Anion.