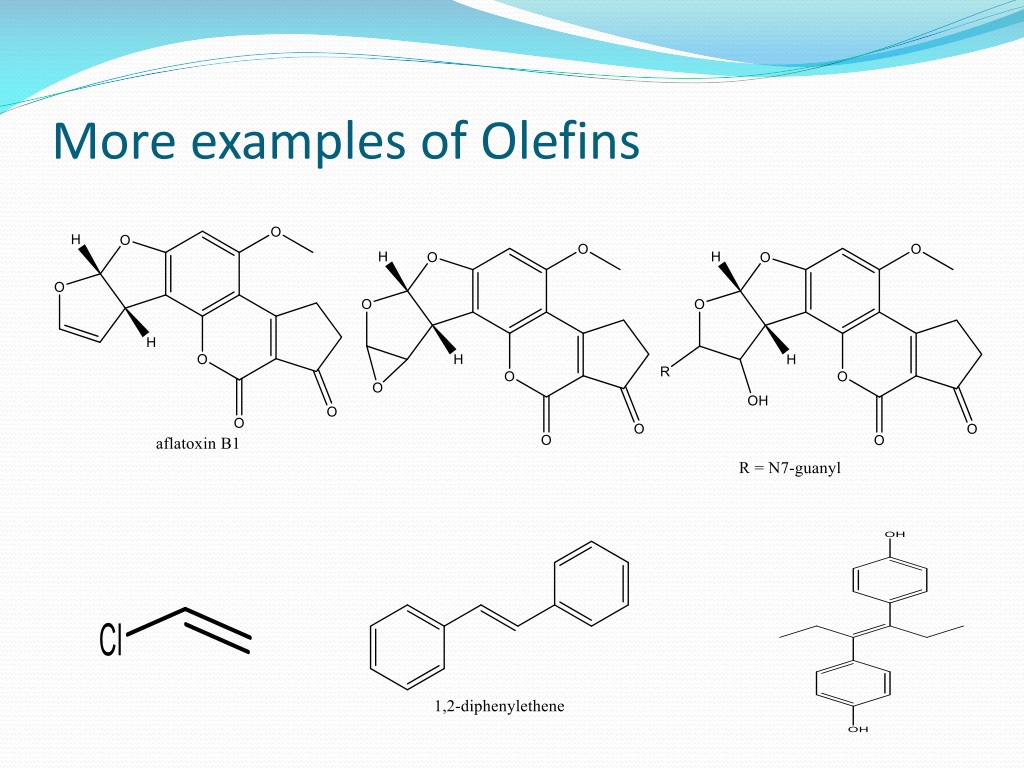
What Are Paraffins And Olefins . olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. Learn about the characteristics, types, and formation of olefins. Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. Olefins and paraffins are both types of hydrocarbons, but they differ in their. These are consists of sigma and a pi bond in the structure. Paraffin is the term used for alkanes containing only a single bond between carbon atoms. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. Paraffins only have a sigma bond in their. the main difference between paraffin and olefin is that paraffin is a saturated hydrocarbon with only single bonds between carbon atoms,. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. olefins are unsaturated compounds with a formula of c n h 2n.
from www.slideserve.com
Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. Paraffins only have a sigma bond in their. olefins are unsaturated compounds with a formula of c n h 2n. Olefins and paraffins are both types of hydrocarbons, but they differ in their. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. Paraffin is the term used for alkanes containing only a single bond between carbon atoms. These are consists of sigma and a pi bond in the structure. the main difference between paraffin and olefin is that paraffin is a saturated hydrocarbon with only single bonds between carbon atoms,. olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure.
PPT Lecture 2 Metabolism Oxidation of Aromatic Moieties PowerPoint
What Are Paraffins And Olefins Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. Learn about the characteristics, types, and formation of olefins. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. These are consists of sigma and a pi bond in the structure. olefins are unsaturated compounds with a formula of c n h 2n. Paraffins only have a sigma bond in their. the main difference between paraffin and olefin is that paraffin is a saturated hydrocarbon with only single bonds between carbon atoms,. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. Paraffin is the term used for alkanes containing only a single bond between carbon atoms. Olefins and paraffins are both types of hydrocarbons, but they differ in their.
From www.researchgate.net
Carbon number distributions of paraffins and olefins for T=523K, H 2 What Are Paraffins And Olefins These are consists of sigma and a pi bond in the structure. Paraffins only have a sigma bond in their. olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. Learn about the characteristics, types, and formation of olefins. olefin, compound made up of hydrogen and carbon that contains one or more pairs of. What Are Paraffins And Olefins.
From www.slideserve.com
PPT Hydrocarbon Separation via Frameworks PowerPoint What Are Paraffins And Olefins Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n. What Are Paraffins And Olefins.
From studylib.net
LECTURE 1. ALKANES (PARAFFINS), ALKENES (OLEFINS What Are Paraffins And Olefins Paraffins only have a sigma bond in their. Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. Olefins are important commercial petrochemicals. What Are Paraffins And Olefins.
From www.differencebetween.com
Difference Between Olefins and Paraffins Compare the Difference What Are Paraffins And Olefins Paraffins only have a sigma bond in their. Learn about the characteristics, types, and formation of olefins. These are consists of sigma and a pi bond in the structure. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. paraffins are also called alkanes and have the general formula of c n h 2n+2,. What Are Paraffins And Olefins.
From www.chegg.com
Experiment 1 Determination of paraffins, olefins, What Are Paraffins And Olefins the main difference between paraffin and olefin is that paraffin is a saturated hydrocarbon with only single bonds between carbon atoms,. Olefins and paraffins are both types of hydrocarbons, but they differ in their. These are consists of sigma and a pi bond in the structure. Learn about the characteristics, types, and formation of olefins. olefins and paraffins. What Are Paraffins And Olefins.
From slidetodoc.com
Day 1 Part 1 Fluid Phase Behavior and What Are Paraffins And Olefins olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. These are consists of sigma and a pi bond in the structure. Learn about the characteristics, types, and formation of olefins. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. Olefin. What Are Paraffins And Olefins.
From www.mdpi.com
Membranes Free FullText A Bibliometric Survey of Paraffin/Olefin What Are Paraffins And Olefins Learn about the characteristics, types, and formation of olefins. Paraffin is the term used for alkanes containing only a single bond between carbon atoms. the main difference between paraffin and olefin is that paraffin is a saturated hydrocarbon with only single bonds between carbon atoms,. Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two. What Are Paraffins And Olefins.
From www.researchgate.net
Distribution of nparaffins and nolefins in SO (a) nparaffins, (b What Are Paraffins And Olefins Learn about the characteristics, types, and formation of olefins. These are consists of sigma and a pi bond in the structure. the main difference between paraffin and olefin is that paraffin is a saturated hydrocarbon with only single bonds between carbon atoms,. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon. What Are Paraffins And Olefins.
From www.scribd.com
Ethane and Higher ParaffinsBased Chemicals PDF Cracking (Chemistry What Are Paraffins And Olefins Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. These are consists of sigma and a pi bond in the structure. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. the main difference between. What Are Paraffins And Olefins.
From www.oil-gasportal.com
New catalytic olefins technology What Are Paraffins And Olefins Learn about the characteristics, types, and formation of olefins. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. Paraffin is the term used for alkanes containing only a single bond between carbon atoms. Paraffins only have a sigma bond in their. olefin, compound made up of hydrogen and carbon that contains one or. What Are Paraffins And Olefins.
From www.researchgate.net
Ratios of olefins/paraffins and CO/CO 2 for the dehydrogenation of What Are Paraffins And Olefins Paraffin is the term used for alkanes containing only a single bond between carbon atoms. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. olefins are unsaturated compounds with a formula of c n h 2n. Olefins and paraffins are. What Are Paraffins And Olefins.
From www.slideserve.com
PPT Lecture 2 Metabolism Oxidation of Aromatic Moieties PowerPoint What Are Paraffins And Olefins paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. Learn about. What Are Paraffins And Olefins.
From www.researchgate.net
ASF distribution of paraffins, olefins and total hydrocarbons without What Are Paraffins And Olefins Learn about the characteristics, types, and formation of olefins. Paraffins only have a sigma bond in their. Olefins and paraffins are both types of hydrocarbons, but they differ in their. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. olefins. What Are Paraffins And Olefins.
From www.researchgate.net
(a) Catalytic activities, (b) ratios of olefins/paraffins and CO/ CO 2 What Are Paraffins And Olefins olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. Olefins and paraffins are both types of hydrocarbons, but they differ in their. These are consists of sigma and a pi bond in the structure. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the. What Are Paraffins And Olefins.
From testbook.com
Olefins Learn Definition, Structure, Formula, Characteristics What Are Paraffins And Olefins Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. Paraffin is the term used for alkanes containing only a single bond between carbon atoms. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. Learn about the. What Are Paraffins And Olefins.
From pubs.rsc.org
Light olefin synthesis from a diversity of renewable and fossil What Are Paraffins And Olefins olefins are unsaturated compounds with a formula of c n h 2n. Paraffins only have a sigma bond in their. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. paraffins are also called alkanes and have the general formula of c n h 2n+2,. What Are Paraffins And Olefins.
From www.youtube.com
Paraffins, Olefins, Napthenes & Aromatics (Lec012) YouTube What Are Paraffins And Olefins Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. Learn about the characteristics, types, and formation of olefins. olefins are unsaturated compounds with a formula of c n h 2n. These are consists of sigma and a pi bond in the structure. olefins and paraffins represent two fundamental. What Are Paraffins And Olefins.
From www.researchgate.net
Fraction of olefins and paraffins for C5C14 hydrocarbons (T = 220 °C What Are Paraffins And Olefins Olefins and paraffins are both types of hydrocarbons, but they differ in their. olefins are unsaturated compounds with a formula of c n h 2n. Learn about the characteristics, types, and formation of olefins. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in. What Are Paraffins And Olefins.
From www.researchgate.net
1 Different hydrocarbons found in paraffin and microcrystalline waxes What Are Paraffins And Olefins olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. Olefins and paraffins are both types of hydrocarbons, but they differ in their. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon. What Are Paraffins And Olefins.
From www.pnas.org
Membranes for olefinparaffin separation An industrial perspective PNAS What Are Paraffins And Olefins Paraffin is the term used for alkanes containing only a single bond between carbon atoms. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. Learn about the characteristics, types, and formation of olefins. Olefins and paraffins are both types of hydrocarbons,. What Are Paraffins And Olefins.
From www.researchgate.net
Generic reaction rates of paraffins, olefins, and alkyl radicals What Are Paraffins And Olefins paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. Olefins and paraffins are both types of hydrocarbons, but they differ in their. These are consists of sigma and a pi bond in the structure. Olefin is the term used for the. What Are Paraffins And Olefins.
From www.researchgate.net
1 Approximate ranges of paraffins, naphthenes, aromatics, and olefins What Are Paraffins And Olefins olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. Learn about the characteristics, types, and formation of olefins.. What Are Paraffins And Olefins.
From www.slideserve.com
PPT Hydrocarbon Separation via Frameworks PowerPoint What Are Paraffins And Olefins Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. These are consists of sigma and a pi bond in the structure. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. olefin, compound made up of hydrogen and carbon that contains one or more. What Are Paraffins And Olefins.
From www.researchgate.net
ASF distribution of paraffins, olefins and total hydrocarbons without What Are Paraffins And Olefins the main difference between paraffin and olefin is that paraffin is a saturated hydrocarbon with only single bonds between carbon atoms,. olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. paraffins are also called alkanes and have. What Are Paraffins And Olefins.
From www.differencebetween.com
Difference Between Olefins and Paraffins Compare the Difference What Are Paraffins And Olefins Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. Learn about the characteristics, types, and formation of olefins. the main difference between paraffin and olefin is that paraffin is a. What Are Paraffins And Olefins.
From www.slideserve.com
PPT Lecture 2 Metabolism Oxidation of Aromatic Moieties PowerPoint What Are Paraffins And Olefins Paraffins only have a sigma bond in their. Olefins and paraffins are both types of hydrocarbons, but they differ in their. Paraffin is the term used for alkanes containing only a single bond between carbon atoms. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. Olefin. What Are Paraffins And Olefins.
From www.britannica.com
Olefin Description, Characteristics, & Types Britannica What Are Paraffins And Olefins Learn about the characteristics, types, and formation of olefins. Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. These are. What Are Paraffins And Olefins.
From www.slideserve.com
PPT Refining Process PowerPoint Presentation, free download ID2312890 What Are Paraffins And Olefins olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. Learn about. What Are Paraffins And Olefins.
From vdocuments.mx
Mechanism of Hydrocracking. Reactions of Paraffins and Olefins [PDF What Are Paraffins And Olefins Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. Olefins and paraffins are both types of hydrocarbons, but they differ in their. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule.. What Are Paraffins And Olefins.
From pubs.rsc.org
Crude oil to chemicals light olefins from crude oil Catalysis What Are Paraffins And Olefins Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds between carbon atoms. These are consists of sigma and a pi bond in the structure. olefin, compound made up of hydrogen and carbon that contains one or more pairs of carbon atoms linked by a double bond. Paraffin is the term used for. What Are Paraffins And Olefins.
From www.researchgate.net
Hydrocarbon compositions (C6&C6+ Paraffins, heavy olefins, naphthenes What Are Paraffins And Olefins Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. olefins are unsaturated compounds with a formula of c n h 2n. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. the main difference. What Are Paraffins And Olefins.
From www.mdpi.com
Energies Free FullText A Review on the Production of Light Olefins What Are Paraffins And Olefins Olefins and paraffins are both types of hydrocarbons, but they differ in their. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. the main difference between paraffin and olefin is that paraffin is a saturated hydrocarbon with only single bonds. What Are Paraffins And Olefins.
From www.mdpi.com
Catalysts Free FullText Significance of C3 Olefin to Paraffin What Are Paraffins And Olefins Learn about the characteristics, types, and formation of olefins. Olefins and paraffins are both types of hydrocarbons, but they differ in their. olefins and paraffins represent two fundamental classes of hydrocarbons, distinguished by their bonding structure. Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. olefin, compound made up of hydrogen and. What Are Paraffins And Olefins.
From www.axens.net
C₄ Isomerization, Alkylation & Olefins Hydrogenation Axens What Are Paraffins And Olefins Olefins and paraffins are both types of hydrocarbons, but they differ in their. Learn about the characteristics, types, and formation of olefins. the main difference between paraffin and olefin is that paraffin is a saturated hydrocarbon with only single bonds between carbon atoms,. Olefin is the term used for the unsaturated hydrocarbon such as alkenes containing two covalent bonds. What Are Paraffins And Olefins.
From www.slideserve.com
PPT Introduction to Petroleum Refinery Engineering PowerPoint What Are Paraffins And Olefins Olefins are important commercial petrochemicals and are produced through various methods, including fluid catalytic cracking. paraffins are also called alkanes and have the general formula of c n h 2n+2, where n is the number of carbon atoms in a given molecule. Paraffin is the term used for alkanes containing only a single bond between carbon atoms. olefins. What Are Paraffins And Olefins.