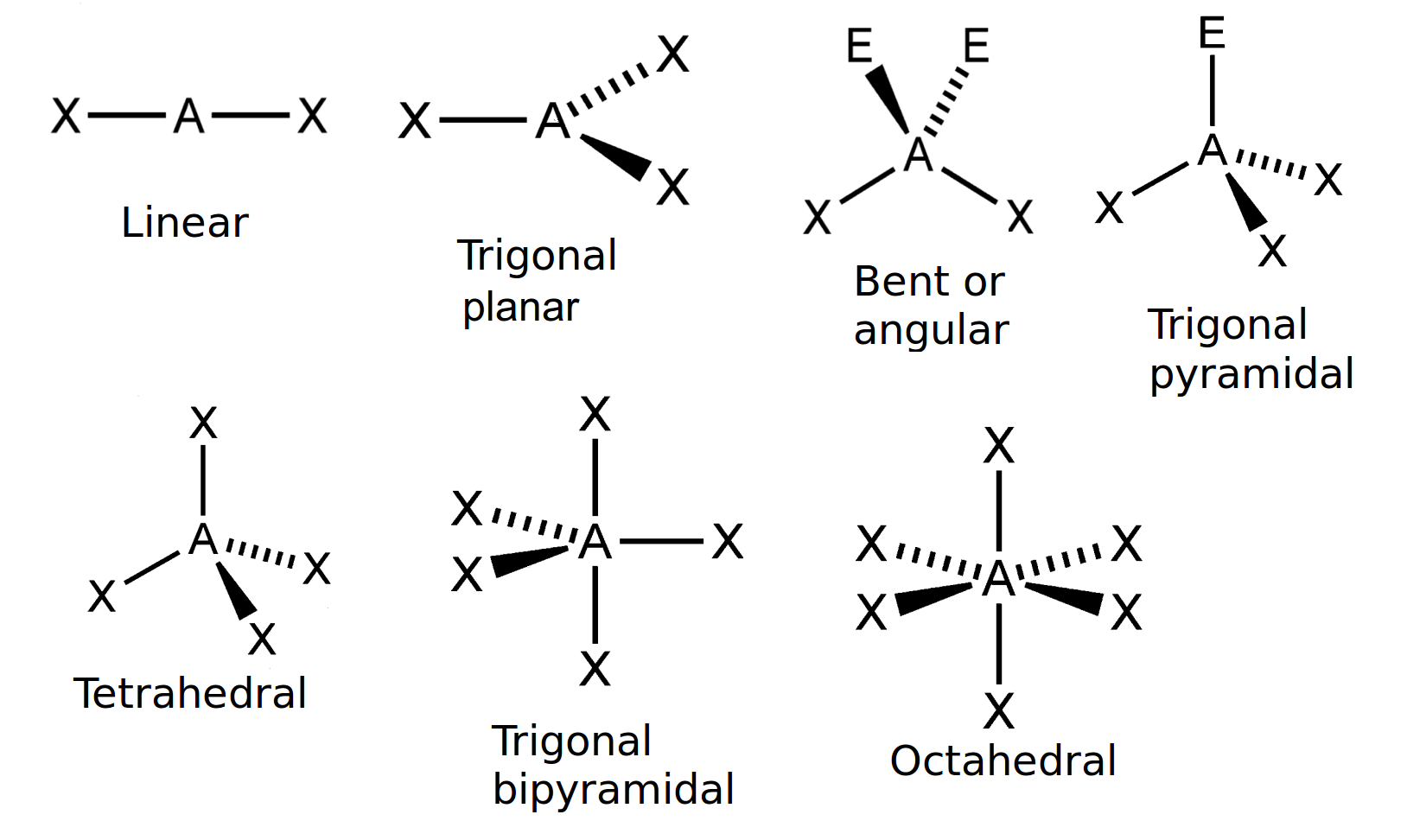
What Is The 3D Molecular Shape Of H2Co . Two regions of electron density around a central atom in a molecule form a linear geometry; The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. Formaldehyde, also known as h2co, has trigonal planar geometry. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. The net dipole moment of. Three regions form a trigonal planar geometry; In this structure, each hydrogen atom. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. This is because it has three regions of electron density.
from www.siyavula.com
Formaldehyde, also known as h2co, has trigonal planar geometry. In this structure, each hydrogen atom. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Three regions form a trigonal planar geometry; Two regions of electron density around a central atom in a molecule form a linear geometry; This is because it has three regions of electron density. The net dipole moment of. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape.
3.2 Molecular shape Atomic combinations Siyavula
What Is The 3D Molecular Shape Of H2Co Formaldehyde, also known as h2co, has trigonal planar geometry. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. In this structure, each hydrogen atom. Two regions of electron density around a central atom in a molecule form a linear geometry; The net dipole moment of. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. This is because it has three regions of electron density. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. Three regions form a trigonal planar geometry; Formaldehyde, also known as h2co, has trigonal planar geometry.
From www.transformationtutoring.com
How To Predict Shape/Molecular Geometry Of A Molecule What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. This is because it has three regions of electron density. Two regions of electron density around a central atom in a molecule form a linear geometry; The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical. What Is The 3D Molecular Shape Of H2Co.
From www.vrogue.co
Vsepr Theory Molecular Shapes Chart Download Printabl vrogue.co What Is The 3D Molecular Shape Of H2Co Three regions form a trigonal planar geometry; The net dipole moment of. Two regions of electron density around a central atom in a molecule form a linear geometry; The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. Formaldehyde, also known as h2co, has trigonal planar geometry. The h2co lewis structure is a representation of the molecular. What Is The 3D Molecular Shape Of H2Co.
From stock.adobe.com
Vetor de Structure of molecules. 3 D formaldehyde (formalin) molecule What Is The 3D Molecular Shape Of H2Co The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. This is because it has three regions of electron density. Two regions of electron density around a central atom in a molecule form a linear geometry; The net dipole moment of. Formaldehyde, also known as h2co, has. What Is The 3D Molecular Shape Of H2Co.
From www.animalia-life.club
Lewis Dot Structure For H2co What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The net dipole moment of. Three regions form a trigonal planar geometry; The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. The molecule h2co, also known as formaldehyde, has. What Is The 3D Molecular Shape Of H2Co.
From www.chegg.com
Solved Question 18 3 pts What is the molecular shape of H2CO What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. Three regions form a trigonal planar geometry; The net dipole moment of. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. Formaldehyde, also known as h2co, has trigonal planar geometry. This is because it has three regions of electron. What Is The 3D Molecular Shape Of H2Co.
From www.showme.com
ShowMe molecular polarity of H2cO What Is The 3D Molecular Shape Of H2Co The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. This is because it has three regions of electron density. Formaldehyde, also known as h2co, has trigonal planar geometry. Two regions of electron density around a central atom in a molecule form a linear geometry; The net dipole moment of. Three regions form a trigonal planar geometry;. What Is The 3D Molecular Shape Of H2Co.
From techiescientist.com
CF4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. Three regions form a trigonal planar geometry; Formaldehyde, also known as h2co, has trigonal planar geometry. In this structure, each hydrogen atom. Two regions of electron density around a central atom in a molecule form a linear geometry; The net dipole moment of.. What Is The 3D Molecular Shape Of H2Co.
From www.siyavula.com
3.2 Molecular shape Atomic combinations Siyavula What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. Formaldehyde, also known as h2co, has trigonal planar geometry. This is because it has three regions of electron density. Three regions form a trigonal planar geometry; In this structure, each. What Is The 3D Molecular Shape Of H2Co.
From www.pngwing.com
Formaldehído compuesto químico composición química química, ángulo What Is The 3D Molecular Shape Of H2Co Formaldehyde, also known as h2co, has trigonal planar geometry. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. In this structure, each hydrogen atom. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. The electron geometry, as well as molecular geometry of. What Is The 3D Molecular Shape Of H2Co.
From www.vectorstock.com
H2co formaldehyde molecule Royalty Free Vector Image What Is The 3D Molecular Shape Of H2Co In this structure, each hydrogen atom. This is because it has three regions of electron density. The net dipole moment of. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Formaldehyde, also known as h2co, has trigonal planar geometry. The molecule h2co, also known as formaldehyde,. What Is The 3D Molecular Shape Of H2Co.
From techiescientist.com
H2O Lewis Structure, Molecular Geometry, and Hybridization What Is The 3D Molecular Shape Of H2Co Formaldehyde, also known as h2co, has trigonal planar geometry. Three regions form a trigonal planar geometry; The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. Two regions of electron density around a central atom in a molecule form a linear geometry; The net dipole moment of. The molecule h2co, also known as. What Is The 3D Molecular Shape Of H2Co.
From www.myxxgirl.com
Co Lewis Structure Geometry And Hybridization My XXX Hot Girl What Is The 3D Molecular Shape Of H2Co In this structure, each hydrogen atom. Three regions form a trigonal planar geometry; The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. Formaldehyde, also known as h2co, has trigonal planar geometry. The net dipole moment of. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the. What Is The 3D Molecular Shape Of H2Co.
From www.ch.imperial.ac.uk
The nature of the C≡S triple bond part 3. Henry Rzepa's Blog Henry What Is The 3D Molecular Shape Of H2Co Two regions of electron density around a central atom in a molecule form a linear geometry; The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. This is because it has three regions of electron density. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. Formaldehyde, also known as. What Is The 3D Molecular Shape Of H2Co.
From animalia-life.club
Ch3cooh Molecular Geometry What Is The 3D Molecular Shape Of H2Co Three regions form a trigonal planar geometry; Two regions of electron density around a central atom in a molecule form a linear geometry; The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Formaldehyde, also known as h2co, has trigonal planar geometry. The molecule h2co, also known. What Is The 3D Molecular Shape Of H2Co.
From techiescientist.com
H2O Lewis Structure, Molecular Geometry, and Hybridization What Is The 3D Molecular Shape Of H2Co This is because it has three regions of electron density. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. The molecule h2co, also known as formaldehyde, has a trigonal. What Is The 3D Molecular Shape Of H2Co.
From draweasy8.blogspot.com
What Is The Molecular Shape Of H2co Draw Easy What Is The 3D Molecular Shape Of H2Co Formaldehyde, also known as h2co, has trigonal planar geometry. This is because it has three regions of electron density. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Two regions of electron density around a central atom in a molecule form a linear geometry; In this. What Is The 3D Molecular Shape Of H2Co.
From www.pinterest.com
H2o 3d Structure Png di 2020 What Is The 3D Molecular Shape Of H2Co The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Two regions of electron density around a. What Is The 3D Molecular Shape Of H2Co.
From www.vrogue.co
H2co3 Lewis Structure Molecular Geometry Hybridizatio vrogue.co What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. This is because it has three regions of electron density. Three regions form a trigonal planar geometry; The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Two regions of. What Is The 3D Molecular Shape Of H2Co.
From www.kompas.com
Notasi VSEPR Pengertian, Rumus, dan Bentuk Molekulnya What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. The net dipole moment of. This is because it has three regions of electron density. The molecule h2co, also known. What Is The 3D Molecular Shape Of H2Co.
From www.myxxgirl.com
Structure Of Methane Ch My XXX Hot Girl What Is The 3D Molecular Shape Of H2Co The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Three regions form a trigonal planar geometry; The net dipole moment of. Two regions of electron density around a central atom in a molecule form a linear geometry; The molecule h2co, also known as formaldehyde, has a. What Is The 3D Molecular Shape Of H2Co.
From news.mit.edu
Taking some of the guesswork out of drug discovery MIT News What Is The 3D Molecular Shape Of H2Co Formaldehyde, also known as h2co, has trigonal planar geometry. This is because it has three regions of electron density. Three regions form a trigonal planar geometry; The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. The net dipole moment. What Is The 3D Molecular Shape Of H2Co.
From mavink.com
Hydrogen Ball And Stick Model What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. In this structure, each hydrogen atom. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Formaldehyde, also known as h2co, has trigonal planar geometry. The net dipole moment of.. What Is The 3D Molecular Shape Of H2Co.
From www.windowssearch-exp.com
SO2 Molecular Geometry Bing images What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. The net dipole moment of. This is because it has three regions of electron density. In this structure, each hydrogen. What Is The 3D Molecular Shape Of H2Co.
From www.transtutors.com
(Get Answer) PART 2 Compounds With 3 Electron Domains Around Central What Is The 3D Molecular Shape Of H2Co Formaldehyde, also known as h2co, has trigonal planar geometry. Three regions form a trigonal planar geometry; This is because it has three regions of electron density. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. In this structure, each hydrogen atom. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per. What Is The 3D Molecular Shape Of H2Co.
From stock.adobe.com
Illustration of 3d molecular structure, ball and stick molecule model What Is The 3D Molecular Shape Of H2Co This is because it has three regions of electron density. Three regions form a trigonal planar geometry; Two regions of electron density around a central atom in a molecule form a linear geometry; Formaldehyde, also known as h2co, has trigonal planar geometry. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound. What Is The 3D Molecular Shape Of H2Co.
From techiescientist.com
H2CO Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram What Is The 3D Molecular Shape Of H2Co This is because it has three regions of electron density. In this structure, each hydrogen atom. The net dipole moment of. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula. What Is The 3D Molecular Shape Of H2Co.
From jeopardylabs.com
BHS Chemistry Spring 2023 DPM Review Jeopardy Template What Is The 3D Molecular Shape Of H2Co Three regions form a trigonal planar geometry; In this structure, each hydrogen atom. The net dipole moment of. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. This is because it has three. What Is The 3D Molecular Shape Of H2Co.
From techiescientist.com
CH2O Lewis Structure, Molecular Geometry, and Hybridization What Is The 3D Molecular Shape Of H2Co In this structure, each hydrogen atom. This is because it has three regions of electron density. Formaldehyde, also known as h2co, has trigonal planar geometry. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. The h2co lewis structure is. What Is The 3D Molecular Shape Of H2Co.
From www.youtube.com
H2CO Molecular Geometry, Bond Angles & Electron Geometry (Formaldehyde What Is The 3D Molecular Shape Of H2Co The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Three regions form a trigonal planar geometry; In this structure, each hydrogen atom. Two regions of electron density around a central atom in a molecule form a linear geometry; The electron geometry, as well as molecular geometry. What Is The 3D Molecular Shape Of H2Co.
From draweasy9.blogspot.com
Pcl3 Electron Geometry And Molecular Geometry Drawing Easy What Is The 3D Molecular Shape Of H2Co The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. The net dipole moment of. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. This is. What Is The 3D Molecular Shape Of H2Co.
From scientifictutor.org
Chem Molecular Shape (Molecular Geometry) Scientific Tutor What Is The 3D Molecular Shape Of H2Co The net dipole moment of. Formaldehyde, also known as h2co, has trigonal planar geometry. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. In this structure, each hydrogen atom. The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. The electron geometry, as. What Is The 3D Molecular Shape Of H2Co.
From www.aceorganicchem.com
Molecular Geometry of NO2 [with video and free study guide] What Is The 3D Molecular Shape Of H2Co The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. Three regions form a trigonal planar geometry; Two regions of electron density around a central atom in a molecule form a linear geometry; The molecule h2co, also known as formaldehyde, has a trigonal planar molecular shape. Formaldehyde,. What Is The 3D Molecular Shape Of H2Co.
From manuallistcantabank.z21.web.core.windows.net
H2o Molecular Diagram What Is The 3D Molecular Shape Of H2Co This is because it has three regions of electron density. The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. Two regions of electron density around a central atom in. What Is The 3D Molecular Shape Of H2Co.
From ar.inspiredpencil.com
O2 2 Lewis Structure What Is The 3D Molecular Shape Of H2Co The electron geometry, as well as molecular geometry of h2co, is trigonal planar as per vsepr theory. Three regions form a trigonal planar geometry; This is because it has three regions of electron density. The net dipole moment of. Two regions of electron density around a central atom in a molecule form a linear geometry; The molecule h2co, also known. What Is The 3D Molecular Shape Of H2Co.
From draweasy8.blogspot.com
H2co Lewis Structure Formal Charge Draw Easy What Is The 3D Molecular Shape Of H2Co The h2co lewis structure is a representation of the molecular structure of formaldehyde, which is a chemical compound with the chemical formula h2co. The net dipole moment of. In this structure, each hydrogen atom. Three regions form a trigonal planar geometry; This is because it has three regions of electron density. Two regions of electron density around a central atom. What Is The 3D Molecular Shape Of H2Co.