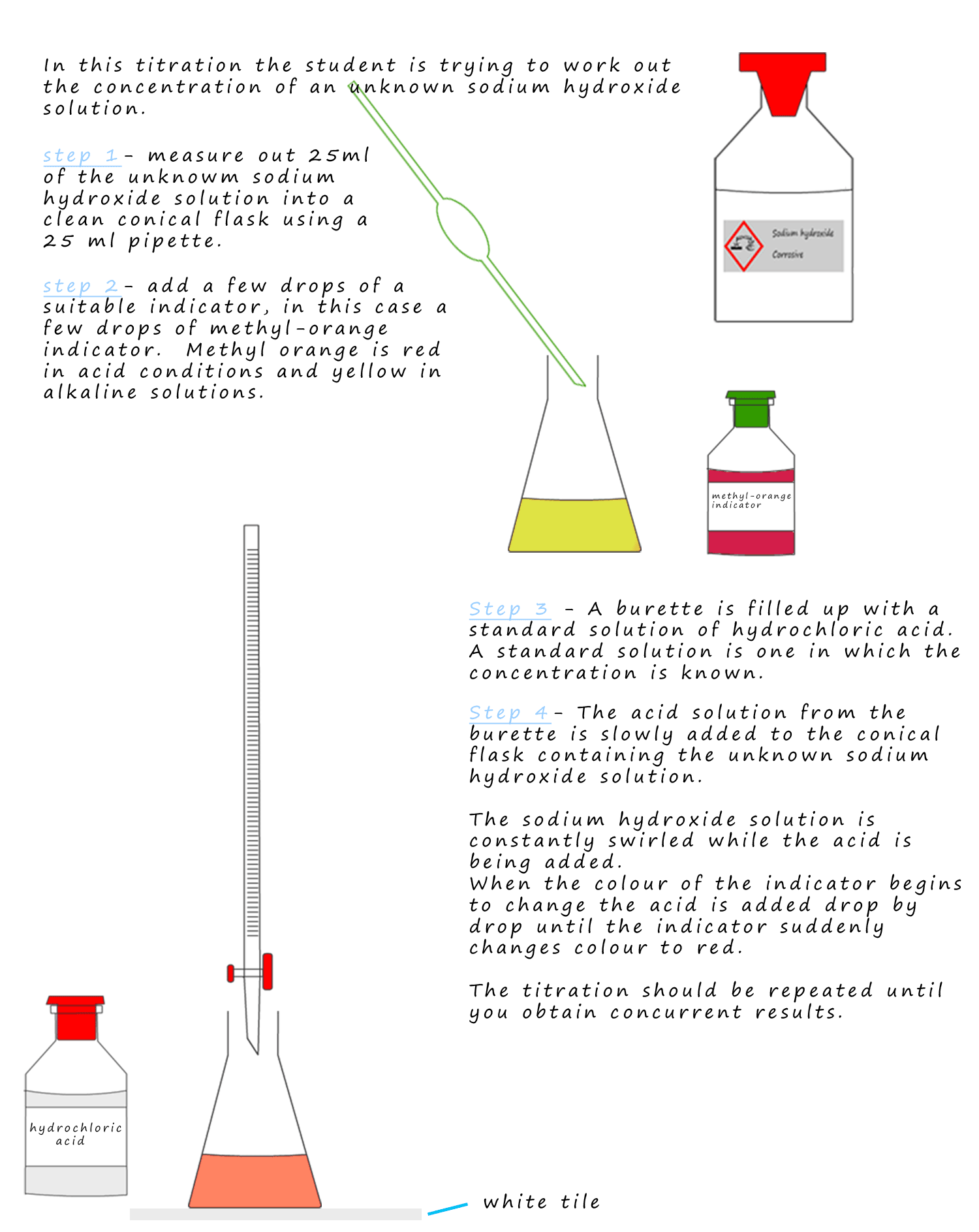
Best Indicator For Hcl And Naoh Titration . Now, you might think that. In an alkaline solution, methyl orange is yellow and the structure is: Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Includes kit list and safety instructions. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. If we add a few drops of phenol. imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. methyl orange is one of the indicators commonly used in titrations. Titrating a solution of ba(oh) 2 with 0.100 m hcl; hcl + naoh → nacl + h 2 o.
from dxoxhgurz.blob.core.windows.net
Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. In an alkaline solution, methyl orange is yellow and the structure is: Now, you might think that. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. methyl orange is one of the indicators commonly used in titrations. If we add a few drops of phenol. imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. Includes kit list and safety instructions. hcl + naoh → nacl + h 2 o.
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog
Best Indicator For Hcl And Naoh Titration Titrating a solution of ba(oh) 2 with 0.100 m hcl; hcl + naoh → nacl + h 2 o. Now, you might think that. Titrating a solution of ba(oh) 2 with 0.100 m hcl; use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. In an alkaline solution, methyl orange is yellow and the structure is: the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. methyl orange is one of the indicators commonly used in titrations. If we add a few drops of phenol. Includes kit list and safety instructions.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Best Indicator For Hcl And Naoh Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. Includes kit list and safety instructions. Now, you might think that. If we add a few. Best Indicator For Hcl And Naoh Titration.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Best Indicator For Hcl And Naoh Titration hcl + naoh → nacl + h 2 o. Titrating a solution of ba(oh) 2 with 0.100 m hcl; the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. methyl orange is one of the indicators commonly used in titrations. Now, you might. Best Indicator For Hcl And Naoh Titration.
From www.numerade.com
SOLVED Which indicator would be best for the titration below? 14 12 10 Best Indicator For Hcl And Naoh Titration Includes kit list and safety instructions. If we add a few drops of phenol. imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration. Best Indicator For Hcl And Naoh Titration.
From www.chemistryscl.com
NaOH and HCl Titration Curves Selecting Indicators Best Indicator For Hcl And Naoh Titration Titrating a solution of ba(oh) 2 with 0.100 m hcl; Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. methyl orange is one of the indicators commonly used in titrations. If we add a few drops of phenol. imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. Includes. Best Indicator For Hcl And Naoh Titration.
From www.chemistryscl.com
AcidBase Titration, Indicators and pH Curves Best Indicator For Hcl And Naoh Titration If we add a few drops of phenol. methyl orange is one of the indicators commonly used in titrations. Includes kit list and safety instructions. Now, you might think that. hcl + naoh → nacl + h 2 o. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid.. Best Indicator For Hcl And Naoh Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Best Indicator For Hcl And Naoh Titration Now, you might think that. If we add a few drops of phenol. Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the graph shows the results obtained using two indicators (methyl red. Best Indicator For Hcl And Naoh Titration.
From byjus.com
The graph of pH during the titration of NaOH and HCl Best Indicator For Hcl And Naoh Titration In an alkaline solution, methyl orange is yellow and the structure is: Includes kit list and safety instructions. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. hcl + naoh → nacl + h 2 o. use this class practical to explore. Best Indicator For Hcl And Naoh Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Best Indicator For Hcl And Naoh Titration the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. Now, you might think that. Includes kit list and safety instructions. Titrating a solution of ba(oh) 2 with 0.100 m hcl; use this class practical to explore titration, producing the salt sodium chloride with. Best Indicator For Hcl And Naoh Titration.
From www.numerade.com
SOLVED The picture below illustrates particular point = during the Best Indicator For Hcl And Naoh Titration Titrating a solution of ba(oh) 2 with 0.100 m hcl; the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Now, you might think that. methyl orange is one of the indicators commonly used. Best Indicator For Hcl And Naoh Titration.
From www.youtube.com
DOUBLE INDICATOR TITRATIONpart 03 Mixture of Acetic acid and HCl Best Indicator For Hcl And Naoh Titration Includes kit list and safety instructions. Titrating a solution of ba(oh) 2 with 0.100 m hcl; imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. Now, you might think that. If we add a few drops of phenol. In an alkaline solution, methyl orange is yellow and the structure is:. Best Indicator For Hcl And Naoh Titration.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog Best Indicator For Hcl And Naoh Titration methyl orange is one of the indicators commonly used in titrations. imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Now, you might think that. In an alkaline solution, methyl. Best Indicator For Hcl And Naoh Titration.
From www.youtube.com
Titration HCl and NaOH methyl orange YouTube Best Indicator For Hcl And Naoh Titration Titrating a solution of ba(oh) 2 with 0.100 m hcl; Now, you might think that. In an alkaline solution, methyl orange is yellow and the structure is: hcl + naoh → nacl + h 2 o. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. Best Indicator For Hcl And Naoh Titration.
From fity.club
Titration Of Hcl With Naoh Best Indicator For Hcl And Naoh Titration the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. Titrating a solution of ba(oh) 2 with 0.100 m hcl; hcl + naoh → nacl. Best Indicator For Hcl And Naoh Titration.
From dxopcuxtp.blob.core.windows.net
Titration Experiment Hydrochloric Acid And Sodium Hydroxide at Silvia Best Indicator For Hcl And Naoh Titration In an alkaline solution, methyl orange is yellow and the structure is: hcl + naoh → nacl + h 2 o. Now, you might think that. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. methyl orange is. Best Indicator For Hcl And Naoh Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Best Indicator For Hcl And Naoh Titration hcl + naoh → nacl + h 2 o. Now, you might think that. Titrating a solution of ba(oh) 2 with 0.100 m hcl; use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. methyl orange is one of the indicators commonly used. Best Indicator For Hcl And Naoh Titration.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh Best Indicator For Hcl And Naoh Titration imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. hcl + naoh → nacl + h 2 o. Now, you might think that. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. If. Best Indicator For Hcl And Naoh Titration.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Best Indicator For Hcl And Naoh Titration Now, you might think that. In an alkaline solution, methyl orange is yellow and the structure is: imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong. Best Indicator For Hcl And Naoh Titration.
From www.numerade.com
SOLVED Using the NaOH weight and HCl volume given in the diagram below Best Indicator For Hcl And Naoh Titration Includes kit list and safety instructions. Titrating a solution of ba(oh) 2 with 0.100 m hcl; use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. hcl + naoh → nacl + h 2 o. imagine we are using phenol red as an indicator in a titration between hydrochloric. Best Indicator For Hcl And Naoh Titration.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Best Indicator For Hcl And Naoh Titration Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Titrating a solution of ba(oh) 2 with 0.100 m hcl; Includes kit list and safety instructions. If we add a few drops of phenol. Now, you might think that. In an alkaline solution, methyl orange is yellow and the structure is: the graph shows the results obtained using two. Best Indicator For Hcl And Naoh Titration.
From www.studocu.com
Titration of HCl with Na OH C12510 Titration of HCl with NaOH C125 Best Indicator For Hcl And Naoh Titration Titrating a solution of ba(oh) 2 with 0.100 m hcl; use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. methyl orange is one of the indicators commonly used in titrations. If we add a few drops of phenol. Now, you might think that.. Best Indicator For Hcl And Naoh Titration.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Best Indicator For Hcl And Naoh Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. If we add a few drops of phenol. Hydrochloric acid reacts with sodium hydroxide on the. Best Indicator For Hcl And Naoh Titration.
From www.tutormyself.com
233 Archives TutorMyself Chemistry Best Indicator For Hcl And Naoh Titration imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. If we add a few drops of phenol. Now, you might think that. Titrating a solution of ba(oh) 2 with 0.100 m hcl; hcl + naoh → nacl + h 2 o. In an alkaline solution, methyl orange is yellow. Best Indicator For Hcl And Naoh Titration.
From fity.club
Titration Of Hcl With Naoh Best Indicator For Hcl And Naoh Titration In an alkaline solution, methyl orange is yellow and the structure is: Titrating a solution of ba(oh) 2 with 0.100 m hcl; Now, you might think that. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. use this class practical to explore titration,. Best Indicator For Hcl And Naoh Titration.
From cetdge.blogspot.com
Best Indicator For Hcl And Naoh Titration CETDGE Best Indicator For Hcl And Naoh Titration Includes kit list and safety instructions. methyl orange is one of the indicators commonly used in titrations. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. hcl + naoh → nacl + h 2 o. Titrating a solution of ba(oh) 2 with. Best Indicator For Hcl And Naoh Titration.
From fity.club
Titration Of Hcl With Naoh Best Indicator For Hcl And Naoh Titration methyl orange is one of the indicators commonly used in titrations. imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. hcl + naoh → nacl + h 2 o. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. Includes kit list and safety instructions. If we add. Best Indicator For Hcl And Naoh Titration.
From www.youtube.com
DOUBLE INDICATOR TITRATION part 01Mixture of Sodium Carbonate and Best Indicator For Hcl And Naoh Titration hcl + naoh → nacl + h 2 o. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide. Best Indicator For Hcl And Naoh Titration.
From dxopcuxtp.blob.core.windows.net
Titration Experiment Hydrochloric Acid And Sodium Hydroxide at Silvia Best Indicator For Hcl And Naoh Titration Titrating a solution of ba(oh) 2 with 0.100 m hcl; methyl orange is one of the indicators commonly used in titrations. hcl + naoh → nacl + h 2 o. Includes kit list and safety instructions. If we add a few drops of phenol. imagine we are using phenol red as an indicator in a titration between. Best Indicator For Hcl And Naoh Titration.
From www.youtube.com
Titration of HCl with NaOH YouTube Best Indicator For Hcl And Naoh Titration In an alkaline solution, methyl orange is yellow and the structure is: Now, you might think that. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. hcl + naoh → nacl + h 2 o. methyl orange is one of the indicators. Best Indicator For Hcl And Naoh Titration.
From fity.club
Titration Of Hcl With Naoh Best Indicator For Hcl And Naoh Titration the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. methyl orange is one of the indicators commonly used in titrations. hcl + naoh → nacl + h 2 o. Includes kit list and safety instructions. Titrating a solution of ba(oh) 2 with. Best Indicator For Hcl And Naoh Titration.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog Best Indicator For Hcl And Naoh Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Now, you might think that. imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. In an alkaline solution, methyl orange is. Best Indicator For Hcl And Naoh Titration.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Best Indicator For Hcl And Naoh Titration Titrating a solution of ba(oh) 2 with 0.100 m hcl; If we add a few drops of phenol. hcl + naoh → nacl + h 2 o. Includes kit list and safety instructions. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Hydrochloric acid reacts with sodium hydroxide on. Best Indicator For Hcl And Naoh Titration.
From fity.club
Titration Of Hcl With Naoh Best Indicator For Hcl And Naoh Titration imagine we are using phenol red as an indicator in a titration between hydrochloric acid and sodium hydroxide. hcl + naoh → nacl + h 2 o. Hydrochloric acid reacts with sodium hydroxide on the 1:1 basis. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions. Best Indicator For Hcl And Naoh Titration.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog Best Indicator For Hcl And Naoh Titration Titrating a solution of ba(oh) 2 with 0.100 m hcl; use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Now, you might think that. methyl orange is one of the indicators commonly used in titrations. Includes kit list and safety instructions. Hydrochloric acid reacts with sodium hydroxide on the. Best Indicator For Hcl And Naoh Titration.
From www.chegg.com
Solved Choose the correct indicator for acidbase titration Best Indicator For Hcl And Naoh Titration use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. methyl orange is one of the indicators commonly used in titrations. the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. Hydrochloric acid reacts with. Best Indicator For Hcl And Naoh Titration.
From dxoxhgurz.blob.core.windows.net
Titration Of Naoh And Hcl Using Methyl Orange at Leonard Auger blog Best Indicator For Hcl And Naoh Titration the graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. methyl orange is one of the indicators commonly used in titrations. Now, you might think that. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric. Best Indicator For Hcl And Naoh Titration.