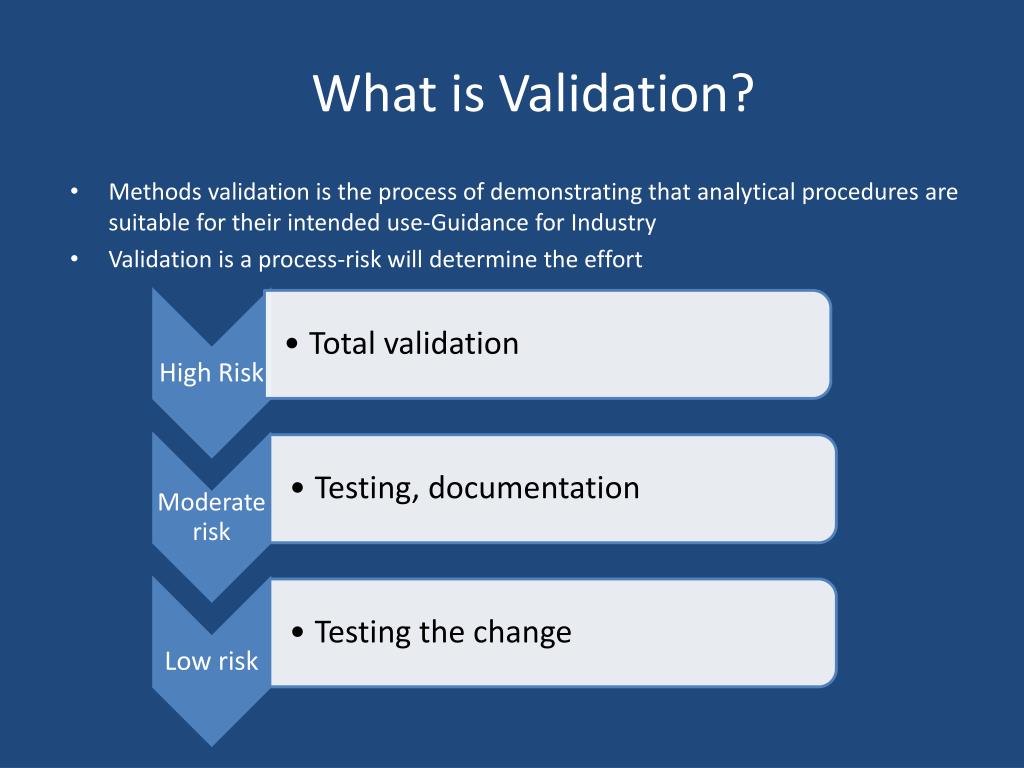
Test Method Validation Gage R&R . Some ways of performing a test method validation include (but is not limited to): What’s the point in weighing yourself? Why you should perform an iq. Test method validation, measurement systems, and gauge r&r. Develop the test method and criteria for pass or fail. Food and drug administration (fda) issued the guidance on test method. Document the test method and criteria. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. The experiment is design to determine how much variation. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Test method validation is a broader term. Confirm that the gage r&r in the study is close to 100 percent. Minitab statistical software offers many types of tools that you can use to assess your measurement system, including: A gage r&r study is a designed experiment to study the variation in measurement results.
from www.slideserve.com
Why you should perform an iq. Test method validation, measurement systems, and gauge r&r. The experiment is design to determine how much variation. Develop the test method and criteria for pass or fail. What’s the point in weighing yourself? A gage r&r study is a designed experiment to study the variation in measurement results. Test method validation is a broader term. Food and drug administration (fda) issued the guidance on test method. Some ways of performing a test method validation include (but is not limited to): Confirm that the gage r&r in the study is close to 100 percent.
PPT A StepbyStep Guide for Method Validation PowerPoint
Test Method Validation Gage R&R Confirm that the gage r&r in the study is close to 100 percent. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Some ways of performing a test method validation include (but is not limited to): Food and drug administration (fda) issued the guidance on test method. Test method validation, measurement systems, and gauge r&r. What’s the point in weighing yourself? The experiment is design to determine how much variation. Why you should perform an iq. Confirm that the gage r&r in the study is close to 100 percent. Develop the test method and criteria for pass or fail. Test method validation is a broader term. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. Minitab statistical software offers many types of tools that you can use to assess your measurement system, including: Document the test method and criteria. A gage r&r study is a designed experiment to study the variation in measurement results.
From www.slideserve.com
PPT Gage R&R PowerPoint Presentation, free download ID6591146 Test Method Validation Gage R&R Test method validation is a broader term. Minitab statistical software offers many types of tools that you can use to assess your measurement system, including: Why you should perform an iq. Test method validation, measurement systems, and gauge r&r. Some ways of performing a test method validation include (but is not limited to): This type of test method validation (tmv). Test Method Validation Gage R&R.
From www.sifo-medical.com
Test Method Validation of Continuous Destructive Measurements (Gage Test Method Validation Gage R&R What’s the point in weighing yourself? Develop the test method and criteria for pass or fail. Test method validation is a broader term. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. Test the test method and criteria (the operational definition) with some test. Test Method Validation Gage R&R.
From www.researchgate.net
Results of Gage R&R with ANOVA method on the input amplitude signals Test Method Validation Gage R&R Minitab statistical software offers many types of tools that you can use to assess your measurement system, including: Confirm that the gage r&r in the study is close to 100 percent. What’s the point in weighing yourself? Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). This type of test method. Test Method Validation Gage R&R.
From www.researchgate.net
Gage R&R study Two way ANOVA Method Improved Results Download Test Method Validation Gage R&R Confirm that the gage r&r in the study is close to 100 percent. Minitab statistical software offers many types of tools that you can use to assess your measurement system, including: Develop the test method and criteria for pass or fail. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Document. Test Method Validation Gage R&R.
From www.kabdwalbook.com
Practical Attribute and Variable Measurement Systems Analysis (MSA) A Test Method Validation Gage R&R Document the test method and criteria. A gage r&r study is a designed experiment to study the variation in measurement results. Some ways of performing a test method validation include (but is not limited to): Test method validation is a broader term. This type of test method validation (tmv) is known as gage r&r and might be the best way. Test Method Validation Gage R&R.
From www.qualitydigest.com
Gauge R&R for Engineering Drawing Services Quality Digest Test Method Validation Gage R&R Food and drug administration (fda) issued the guidance on test method. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. Test method validation is a broader term. Develop the test method and criteria for pass or fail. The experiment is design to determine how. Test Method Validation Gage R&R.
From www.chegg.com
Solved Suppose you conduct a Gage R&R Study using 10 parts, Test Method Validation Gage R&R Why you should perform an iq. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Confirm that the gage r&r in the study is close to 100 percent. Food and drug administration (fda) issued the guidance on test method. Test method validation, measurement systems, and gauge r&r. This type of test. Test Method Validation Gage R&R.
From www.sifo-medical.com
Test Method Validation (TMV) in Medical Device Manufacturing Test Method Validation Gage R&R Some ways of performing a test method validation include (but is not limited to): Food and drug administration (fda) issued the guidance on test method. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). A gage r&r study is a designed experiment to study the variation in measurement results. Document the. Test Method Validation Gage R&R.
From slideplayer.com
Gage R&R. ppt download Test Method Validation Gage R&R A gage r&r study is a designed experiment to study the variation in measurement results. What’s the point in weighing yourself? Confirm that the gage r&r in the study is close to 100 percent. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. The. Test Method Validation Gage R&R.
From www.goskills.com
The Basics of Gage R&R GoSkills Test Method Validation Gage R&R Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Test method validation, measurement systems, and gauge r&r. A gage r&r study is a designed experiment to study the variation in measurement results. Document the test method and criteria. The experiment is design to determine how much variation. What’s the point in. Test Method Validation Gage R&R.
From polymerprocessing.blogspot.com
4. Gage repeatability & reproducibility (R&R) test Validation Test Method Validation Gage R&R Test method validation, measurement systems, and gauge r&r. Document the test method and criteria. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Why you should. Test Method Validation Gage R&R.
From www.youtube.com
Gage R&R in Medical Device Production Explained Simply! YouTube Test Method Validation Gage R&R Document the test method and criteria. Confirm that the gage r&r in the study is close to 100 percent. Test method validation is a broader term. Food and drug administration (fda) issued the guidance on test method. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Test method validation, measurement systems,. Test Method Validation Gage R&R.
From www.automotivequal.com
What is Gage R&R 📏 and why is it crucial for accurate measurements? Test Method Validation Gage R&R The experiment is design to determine how much variation. Confirm that the gage r&r in the study is close to 100 percent. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Minitab statistical software offers many types of tools that you can use to assess your measurement system, including: Develop the. Test Method Validation Gage R&R.
From sixsigmastudyguide.com
Gage Repeatability and Reproducibility (R&R) Test Method Validation Gage R&R A gage r&r study is a designed experiment to study the variation in measurement results. Why you should perform an iq. Test method validation, measurement systems, and gauge r&r. Some ways of performing a test method validation include (but is not limited to): Develop the test method and criteria for pass or fail. Test the test method and criteria (the. Test Method Validation Gage R&R.
From www.scribd.com
Gage R&R Tool Average and Range Method (Control Chart Method, Xbar Test Method Validation Gage R&R A gage r&r study is a designed experiment to study the variation in measurement results. Minitab statistical software offers many types of tools that you can use to assess your measurement system, including: Test method validation, measurement systems, and gauge r&r. Document the test method and criteria. Food and drug administration (fda) issued the guidance on test method. What’s the. Test Method Validation Gage R&R.
From slideplayer.com
Gage R&R. ppt download Test Method Validation Gage R&R The experiment is design to determine how much variation. What’s the point in weighing yourself? Why you should perform an iq. Some ways of performing a test method validation include (but is not limited to): Develop the test method and criteria for pass or fail. Test the test method and criteria (the operational definition) with some test samples (perform a. Test Method Validation Gage R&R.
From www.sifo-medical.com
Conducting a Gage R&R Study with Minitab (Explained with a Practical Test Method Validation Gage R&R What’s the point in weighing yourself? Food and drug administration (fda) issued the guidance on test method. Minitab statistical software offers many types of tools that you can use to assess your measurement system, including: Document the test method and criteria. Test method validation is a broader term. A gage r&r study is a designed experiment to study the variation. Test Method Validation Gage R&R.
From spa.myservername.com
Guía definitiva de pruebas de validación Otro Test Method Validation Gage R&R Document the test method and criteria. Confirm that the gage r&r in the study is close to 100 percent. Some ways of performing a test method validation include (but is not limited to): A gage r&r study is a designed experiment to study the variation in measurement results. What’s the point in weighing yourself? The experiment is design to determine. Test Method Validation Gage R&R.
From templates.udlvirtual.edu.pe
Free Gage Rr Template Printable Templates Test Method Validation Gage R&R Test method validation is a broader term. Document the test method and criteria. The experiment is design to determine how much variation. What’s the point in weighing yourself? Food and drug administration (fda) issued the guidance on test method. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what. Test Method Validation Gage R&R.
From slideplayer.com
Gage R&R. ppt download Test Method Validation Gage R&R Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Some ways of performing a test method validation include (but is not limited to): Test method validation is a broader term. Test method validation, measurement systems, and gauge r&r. What’s the point in weighing yourself? Food and drug administration (fda) issued the. Test Method Validation Gage R&R.
From leansigmacorporation.com
Variable Gage R&R with Minitab Lean Sigma Corporation Test Method Validation Gage R&R Confirm that the gage r&r in the study is close to 100 percent. Test method validation is a broader term. Develop the test method and criteria for pass or fail. Document the test method and criteria. Food and drug administration (fda) issued the guidance on test method. Minitab statistical software offers many types of tools that you can use to. Test Method Validation Gage R&R.
From measurlink.com
Gage R&R MeasurLink Test Method Validation Gage R&R The experiment is design to determine how much variation. Test method validation, measurement systems, and gauge r&r. A gage r&r study is a designed experiment to study the variation in measurement results. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Develop the test method and criteria for pass or fail.. Test Method Validation Gage R&R.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Test Method Validation Gage R&R Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). The experiment is design to determine how much variation. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. A gage r&r study is a designed experiment. Test Method Validation Gage R&R.
From quality-one.com
MSA Measurement System Analysis QualityOne Test Method Validation Gage R&R Why you should perform an iq. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). A gage r&r study is a designed experiment to study the variation in measurement results. Document the test method and criteria. Some ways of performing a test method validation include (but is not limited to): Confirm. Test Method Validation Gage R&R.
From slideplayer.com
Gage R&R. ppt download Test Method Validation Gage R&R Document the test method and criteria. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Test method validation, measurement systems, and gauge r&r. Confirm that the. Test Method Validation Gage R&R.
From www.youtube.com
Gage R&R Study Presentation YouTube Test Method Validation Gage R&R Some ways of performing a test method validation include (but is not limited to): Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Test method validation is a broader term. A gage r&r study is a designed experiment to study the variation in measurement results. Why you should perform an iq.. Test Method Validation Gage R&R.
From www.qimacros.com
Attribute Gage R&R Study Excel Template Pass Fail Gage Test Method Validation Gage R&R Food and drug administration (fda) issued the guidance on test method. A gage r&r study is a designed experiment to study the variation in measurement results. Test method validation is a broader term. What’s the point in weighing yourself? Document the test method and criteria. Why you should perform an iq. The experiment is design to determine how much variation.. Test Method Validation Gage R&R.
From www.slideserve.com
PPT Test Method Validation & Verification PowerPoint Presentation Test Method Validation Gage R&R Some ways of performing a test method validation include (but is not limited to): Test method validation is a broader term. A gage r&r study is a designed experiment to study the variation in measurement results. Document the test method and criteria. Food and drug administration (fda) issued the guidance on test method. The experiment is design to determine how. Test Method Validation Gage R&R.
From www.researchgate.net
The results of Gage R&R (crossed) Analysis Download Scientific Diagram Test Method Validation Gage R&R This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. What’s the point in weighing yourself? Minitab statistical software offers many types of tools that you can use to assess your measurement system, including: Some ways of performing a test method validation include (but is. Test Method Validation Gage R&R.
From readandgain.com
Gage R&R Study (Variable Data) explained with an example! Test Method Validation Gage R&R The experiment is design to determine how much variation. Why you should perform an iq. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. Some ways of performing a test method validation include (but is not limited to): Confirm that the gage r&r in. Test Method Validation Gage R&R.
From vancouverwool.web.fc2.com
Anova Gage R And R Test Method Validation Gage R&R Food and drug administration (fda) issued the guidance on test method. Some ways of performing a test method validation include (but is not limited to): Test the test method and criteria (the operational definition) with some test samples (perform a gage r&r study). Minitab statistical software offers many types of tools that you can use to assess your measurement system,. Test Method Validation Gage R&R.
From www.muelaner.com
How to do Gage R&R in Excel Test Method Validation Gage R&R Develop the test method and criteria for pass or fail. What’s the point in weighing yourself? Test method validation, measurement systems, and gauge r&r. Food and drug administration (fda) issued the guidance on test method. Some ways of performing a test method validation include (but is not limited to): Test method validation is a broader term. This type of test. Test Method Validation Gage R&R.
From techqualitypedia.com
Gage R & R GRR Gage repeatability and reproducibility Test Method Validation Gage R&R The experiment is design to determine how much variation. Test method validation, measurement systems, and gauge r&r. Why you should perform an iq. Test method validation is a broader term. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. Some ways of performing a. Test Method Validation Gage R&R.
From dokumen.tips
(PDF) Test Method Validation Portfolio... · For the crosshair and Test Method Validation Gage R&R Test method validation, measurement systems, and gauge r&r. Why you should perform an iq. This type of test method validation (tmv) is known as gage r&r and might be the best way to show what tmv is all about. The experiment is design to determine how much variation. A gage r&r study is a designed experiment to study the variation. Test Method Validation Gage R&R.
From www.sifo-medical.com
Test Method Validation of Continuous Destructive Measurements (Gage Test Method Validation Gage R&R Test method validation, measurement systems, and gauge r&r. Document the test method and criteria. Develop the test method and criteria for pass or fail. Test method validation is a broader term. A gage r&r study is a designed experiment to study the variation in measurement results. Food and drug administration (fda) issued the guidance on test method. Test the test. Test Method Validation Gage R&R.