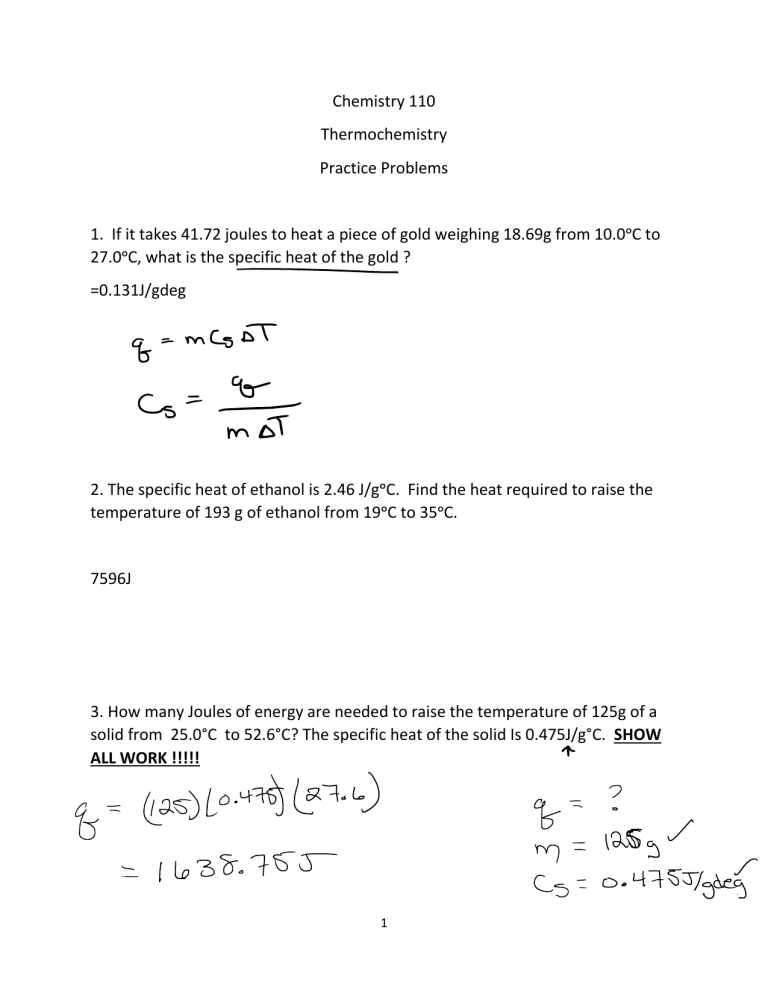
Calorimetry Experiment Hypothesis . Use this equation to calculate the molar enthalpy change for a reaction. determination of enthalpy changes by calorimetry. Students should be able to: calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. calorimetry is the science of measuring heat flow. For example, if you drop a coin into a cup with hot water, the Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: The aims of the experiment are: Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c.
from studylib.net
calorimetry is the science of measuring heat flow. For example, if you drop a coin into a cup with hot water, the to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. Students should be able to: determination of enthalpy changes by calorimetry. The aims of the experiment are: the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:
Calorimetry Experiment and Practice Problems for Virtual Lab Key
Calorimetry Experiment Hypothesis For example, if you drop a coin into a cup with hot water, the the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. Use this equation to calculate the molar enthalpy change for a reaction. determination of enthalpy changes by calorimetry. calorimetry is the science of measuring heat flow. calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; For example, if you drop a coin into a cup with hot water, the Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. Students should be able to: calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. The aims of the experiment are: Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Experiment Hypothesis to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: Students. Calorimetry Experiment Hypothesis.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Experiment Hypothesis calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: Students should be able to: calorimetry is the science of measuring heat flow. Where m is the mass of the substance that has a temperature. Calorimetry Experiment Hypothesis.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 4 SPM Chemistry Calorimetry Experiment Hypothesis Students should be able to: the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: For example, if you drop a coin into a cup with hot water, the to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. calorimetry is the study. Calorimetry Experiment Hypothesis.
From www.linstitute.net
AQA A Level Biology复习笔记5.3.3 Biomass翰林国际教育 Calorimetry Experiment Hypothesis Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. calorimetry is the science of measuring heat flow. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Use this equation to calculate the molar enthalpy. Calorimetry Experiment Hypothesis.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.1.2 Calorimetry翰林国际教育 Calorimetry Experiment Hypothesis Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. determination of enthalpy changes by calorimetry. Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. For example, if you drop a coin into a cup with hot water,. Calorimetry Experiment Hypothesis.
From giobxedsi.blob.core.windows.net
Calorimeter Experiment Calculation at Mabel Ballenger blog Calorimetry Experiment Hypothesis The aims of the experiment are: Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. For example, if you drop a coin into a cup with. Calorimetry Experiment Hypothesis.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Calorimetry Experiment Hypothesis The aims of the experiment are: For example, if you drop a coin into a cup with hot water, the calorimetry is the science of measuring heat flow. the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: Students should be able to: calorimetry is the study of heat transferred in. Calorimetry Experiment Hypothesis.
From studylib.net
experiment 9 Thermochemistry Calorimetry Calorimetry Experiment Hypothesis Use this equation to calculate the molar enthalpy change for a reaction. For example, if you drop a coin into a cup with hot water, the Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. Heat is defined as thermal energy flowing from an object at a higher temperature. Calorimetry Experiment Hypothesis.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Experiment Hypothesis calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. Heat is defined as thermal energy flowing from an object at a higher temperature to one at. Calorimetry Experiment Hypothesis.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Calorimetry Experiment Hypothesis Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: determination of enthalpy changes by calorimetry. For example, if you drop a coin into a cup with hot water, the calorimetry. Calorimetry Experiment Hypothesis.
From www.nagwa.com
Question Video Determining the Suitability of Experimental Apparatus Calorimetry Experiment Hypothesis Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. the diagram. Calorimetry Experiment Hypothesis.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Experiment Hypothesis Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel:. Calorimetry Experiment Hypothesis.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Calorimetry Experiment Hypothesis The aims of the experiment are: For example, if you drop a coin into a cup with hot water, the calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Heat is defined as thermal energy flowing from an object at a higher temperature to one at. Calorimetry Experiment Hypothesis.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Experiment Hypothesis calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. For example, if you drop a coin into a cup with hot water, the calorimetry is the science of measuring heat flow. The aims of the experiment are: Where m is the mass of the substance. Calorimetry Experiment Hypothesis.
From hxekemegs.blob.core.windows.net
Calorimetry Chemistry Experiment at Robert Montague blog Calorimetry Experiment Hypothesis to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. Students should be able to: Use this equation to calculate the molar enthalpy change for a reaction. calorimetry is the science of measuring heat flow. Where m is the mass of the substance that has a temperature change δt and. Calorimetry Experiment Hypothesis.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Calorimetry Experiment Hypothesis Students should be able to: the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. Use this equation to calculate the molar enthalpy change for a reaction. calorimetry is the study of heat. Calorimetry Experiment Hypothesis.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Experiment Hypothesis The aims of the experiment are: determination of enthalpy changes by calorimetry. Use this equation to calculate the molar enthalpy change for a reaction. calorimetry is the science of measuring heat flow. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. Students should be able to: For example,. Calorimetry Experiment Hypothesis.
From sites.google.com
3.2.1 (e) Q=mcΔT Ellesmere OCR A level Chemistry Calorimetry Experiment Hypothesis determination of enthalpy changes by calorimetry. Students should be able to: Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. calorimetry is the science of measuring heat flow. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Calorimetry Experiment Hypothesis.
From www.chegg.com
We are going to use a calorimetry experiment to Calorimetry Experiment Hypothesis For example, if you drop a coin into a cup with hot water, the Use this equation to calculate the molar enthalpy change for a reaction. calorimetry is the science of measuring heat flow. The aims of the experiment are: Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Calorimetry Experiment Hypothesis.
From chem.libretexts.org
6.3 Calorimetry Chemistry LibreTexts Calorimetry Experiment Hypothesis For example, if you drop a coin into a cup with hot water, the The aims of the experiment are: Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. Students should be able to: calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter. Calorimetry Experiment Hypothesis.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Experiment Hypothesis For example, if you drop a coin into a cup with hot water, the calorimetry is the science of measuring heat flow. Students should be able to: to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. the diagram shows a simple calorimetry experiment to measure the heat energy. Calorimetry Experiment Hypothesis.
From www.nagwa.com
Question Video Identifying the Factor That Does Not Need to Be Held Calorimetry Experiment Hypothesis Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: Students should be able to: calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool. Calorimetry Experiment Hypothesis.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Experiment Hypothesis calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; The aims of the experiment are: For example, if you drop a coin into a cup with hot water, the Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. Heat is defined. Calorimetry Experiment Hypothesis.
From hxekemegs.blob.core.windows.net
Calorimetry Chemistry Experiment at Robert Montague blog Calorimetry Experiment Hypothesis Students should be able to: determination of enthalpy changes by calorimetry. the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. For example, if you drop a coin into a cup with. Calorimetry Experiment Hypothesis.
From fyoislawz.blob.core.windows.net
Calorimetry Online Experiment at Catherine blog Calorimetry Experiment Hypothesis calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; The aims of the experiment are: calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Use this equation to calculate the molar enthalpy change for a reaction. calorimetry. Calorimetry Experiment Hypothesis.
From fyokoklvl.blob.core.windows.net
Calorimetry Definition In Chemistry at Patrick McBean blog Calorimetry Experiment Hypothesis calorimetry is the science of measuring heat flow. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. determination of enthalpy changes by calorimetry. The aims. Calorimetry Experiment Hypothesis.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Experiment Hypothesis The aims of the experiment are: calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; Students should be able to: the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: Where m is the mass of the substance that has a temperature change δt and. Calorimetry Experiment Hypothesis.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Experiment Hypothesis determination of enthalpy changes by calorimetry. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. Students should be able to: calorimetry is the science of measuring heat flow.. Calorimetry Experiment Hypothesis.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Experiment Hypothesis calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. For example, if you drop a coin into a cup with hot water, the Use this equation to calculate the molar enthalpy change for. Calorimetry Experiment Hypothesis.
From www.nagwa.com
Question Video Identifying the Trend between the Energy Contained in a Calorimetry Experiment Hypothesis Use this equation to calculate the molar enthalpy change for a reaction. Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Heat is defined as thermal. Calorimetry Experiment Hypothesis.
From studylib.net
Calorimetry Experiment and Practice Problems for Virtual Lab Key Calorimetry Experiment Hypothesis Where m is the mass of the substance that has a temperature change δt and a specific heat capacity c. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. calorimetry the heat change, q, in a reaction is given by the equation q = mcδt;. Calorimetry Experiment Hypothesis.
From giouwyran.blob.core.windows.net
Calorimetry Value Definition at Deborah Pettit blog Calorimetry Experiment Hypothesis calorimetry is the science of measuring heat flow. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. determination of enthalpy changes by calorimetry. The aims of the experiment are: Heat is defined as thermal energy flowing from an object at a higher temperature to. Calorimetry Experiment Hypothesis.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Experiment Hypothesis to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. determination of enthalpy changes by calorimetry. the diagram shows a simple calorimetry experiment to measure the heat energy released from burning fuel: calorimetry the heat change, q, in a reaction is given by the equation q = mcδt;. Calorimetry Experiment Hypothesis.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Calorimetry Experiment Hypothesis Students should be able to: calorimetry the heat change, q, in a reaction is given by the equation q = mcδt; Use this equation to calculate the molar enthalpy change for a reaction. to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. Where m is the mass of the. Calorimetry Experiment Hypothesis.
From www.scribd.com
Calorimetry Experiment PDF Heat Enthalpy Calorimetry Experiment Hypothesis Students should be able to: to experimentally measure the δh δ h values of two reactions using the technique of constant pressure calorimetry. Use this equation to calculate the molar enthalpy change for a reaction. The aims of the experiment are: Where m is the mass of the substance that has a temperature change δt and a specific heat. Calorimetry Experiment Hypothesis.