Copper Electron Configuration Orbital Diagram . The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. Each box represents one orbital, and each arrow indicates one electron. The electron configuration of copper is [ar] 3d10 4s1. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the. The orbital diagram for copper (cu) can be represented as follows: Copper has a unique electron configuration that can be represented using an orbital diagram. For example, the orbital diagram of li can be shown as: Since 1s can only hold two electrons the next 2. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Copper (cu) has an atomic number of 29,. This is a way of showing the electron configuration of the atom. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. In the case of copper, the electron configuration is [ar]. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for.
from www.alamy.com
For example, the orbital diagram of li can be shown as: This is a way of showing the electron configuration of the atom. In the case of copper, the electron configuration is [ar]. This means that copper has a completely filled 3d orbital with 10 electrons and one. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. Copper has a unique electron configuration that can be represented using an orbital diagram. The electron configuration of copper is [ar] 3d10 4s1. Each box represents one orbital, and each arrow indicates one electron. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down.
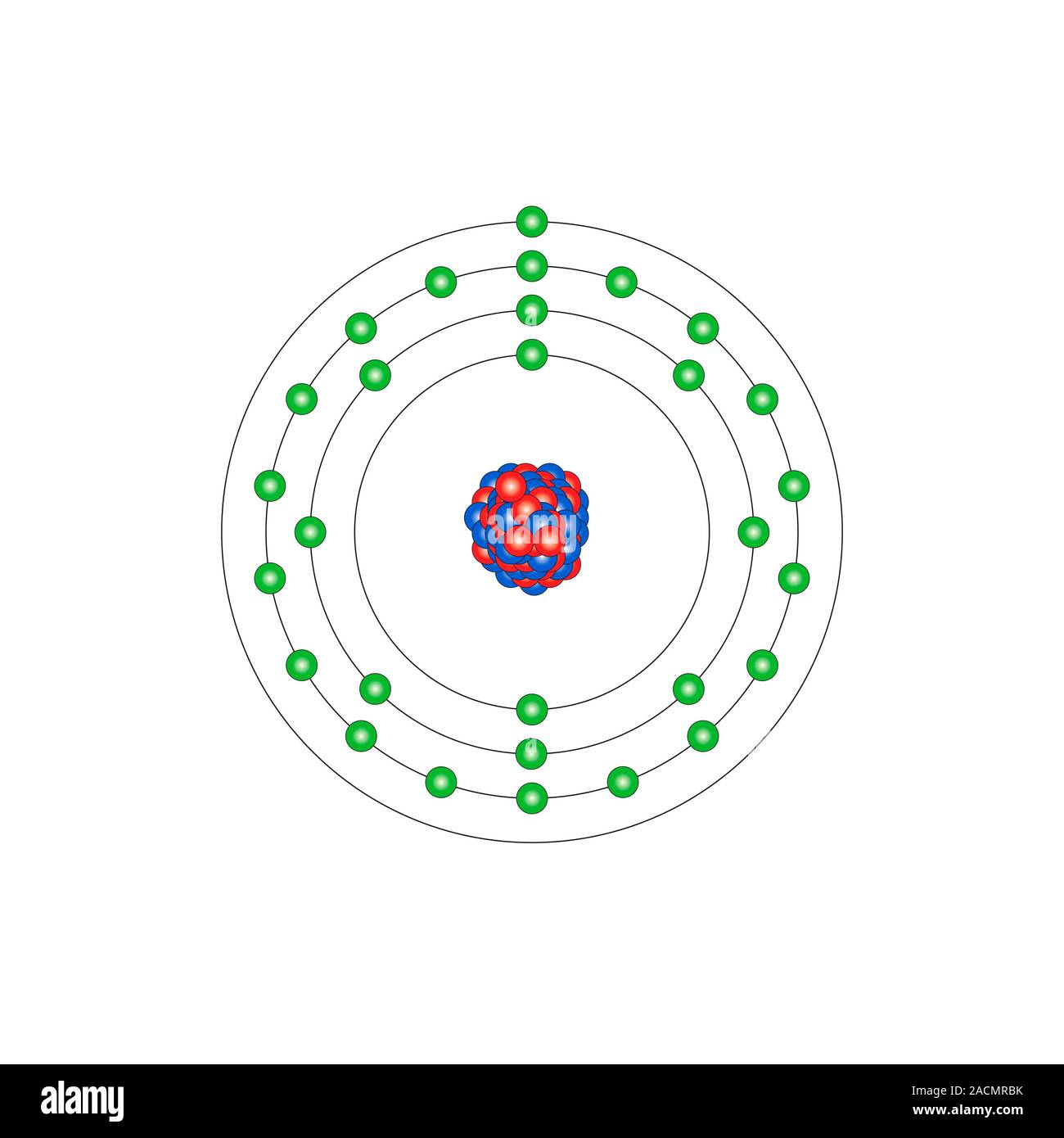
Copper (Cu). Diagram of the nuclear composition and electron
Copper Electron Configuration Orbital Diagram Since 1s can only hold two electrons the next 2. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. This is a way of showing the electron configuration of the atom. The orbital diagram for copper (cu) can be represented as follows: Copper has a unique electron configuration that can be represented using an orbital diagram. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. This means that copper has a completely filled 3d orbital with 10 electrons and one. The electron configuration of copper is [ar] 3d10 4s1. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. In the case of copper, the electron configuration is [ar]. For example, the orbital diagram of li can be shown as: Each box represents one orbital, and each arrow indicates one electron. Since 1s can only hold two electrons the next 2.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Electron Configuration Orbital Diagram The orbital diagram for copper (cu) can be represented as follows: In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Each box represents one orbital, and each arrow indicates one electron. Since 1s can only hold two electrons the next 2. In the case of copper, the electron configuration. Copper Electron Configuration Orbital Diagram.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Orbital Diagram This means that copper has a completely filled 3d orbital with 10 electrons and one. Each box represents one orbital, and each arrow indicates one electron. Copper has a unique electron configuration that can be represented using an orbital diagram. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down.. Copper Electron Configuration Orbital Diagram.
From stewart-switch.com
Orbital Diagram Of Copper Copper Electron Configuration Orbital Diagram An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the. Copper has a unique electron configuration that can be represented using an orbital diagram. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. This is a way of. Copper Electron Configuration Orbital Diagram.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electron Configuration Orbital Diagram To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. Copper (cu) has an atomic number of 29,. The orbital diagram for copper (cu) can be represented as follows: The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. This means that copper has a completely. Copper Electron Configuration Orbital Diagram.
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Electron Configuration Orbital Diagram Copper has a unique electron configuration that can be represented using an orbital diagram. Since 1s can only hold two electrons the next 2. Each box represents one orbital, and each arrow indicates one electron. This is a way of showing the electron configuration of the atom. In writing the electron configuration for copper the first two electrons will go. Copper Electron Configuration Orbital Diagram.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Electron Configuration Orbital Diagram For example, the orbital diagram of li can be shown as: Each box represents one orbital, and each arrow indicates one electron. The electron configuration of copper is [ar] 3d10 4s1. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. The orbital diagram for copper (cu) can be represented as follows:. Copper Electron Configuration Orbital Diagram.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Orbital Diagram Copper (cu) has an atomic number of 29,. For example, the orbital diagram of li can be shown as: The orbital diagram for copper (cu) can be represented as follows: In writing the electron configuration for copper the first two electrons will go in the 1s orbital. To write the orbital diagram for the copper (cu) first we need to. Copper Electron Configuration Orbital Diagram.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Copper Electron Configuration Orbital Diagram For example, the orbital diagram of li can be shown as: This is a way of showing the electron configuration of the atom. Copper has a unique electron configuration that can be represented using an orbital diagram. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. The orbital diagram for copper (cu) can be represented as follows: In writing the electron configuration. Copper Electron Configuration Orbital Diagram.
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Electron Configuration Orbital Diagram For example, the orbital diagram of li can be shown as: The electron configuration of copper is [ar] 3d10 4s1. Each box represents one orbital, and each arrow indicates one electron. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. In the case of copper, the electron configuration is [ar]. Since. Copper Electron Configuration Orbital Diagram.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electron Configuration Orbital Diagram This means that copper has a completely filled 3d orbital with 10 electrons and one. The orbital diagram for copper (cu) can be represented as follows: To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. Each box represents one orbital, and each arrow indicates one electron. The electron configuration of copper. Copper Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Orbital Diagram For Copper Copper Electron Configuration Orbital Diagram An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the. In the case of copper, the electron configuration is [ar]. Copper has a unique electron configuration that can be represented using an orbital diagram. This means that copper has a completely filled 3d orbital with. Copper Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Copper Orbital Diagram Copper Electron Configuration Orbital Diagram In the case of copper, the electron configuration is [ar]. Each box represents one orbital, and each arrow indicates one electron. The orbital diagram for copper (cu) can be represented as follows: This is a way of showing the electron configuration of the atom. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. In orbitals diagrams, the orbitals are shown as boxes,. Copper Electron Configuration Orbital Diagram.
From schematicmaxeywheezle.z21.web.core.windows.net
Orbital Energy Diagram For Copper Copper Electron Configuration Orbital Diagram Each box represents one orbital, and each arrow indicates one electron. In the case of copper, the electron configuration is [ar]. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. For example, the orbital diagram of li can be shown as: Since 1s can only hold two electrons the next 2.. Copper Electron Configuration Orbital Diagram.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Electron Configuration Orbital Diagram In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. For example, the orbital diagram of li can be shown as: In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper has a unique electron configuration that can be represented using an. Copper Electron Configuration Orbital Diagram.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Electron Configuration Orbital Diagram The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. Copper has a unique electron configuration that can be represented using an orbital diagram. The orbital diagram for copper (cu) can be represented as follows: Since 1s can only hold two electrons the next 2. In the case of copper, the electron configuration is. Copper Electron Configuration Orbital Diagram.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Copper Electron Configuration Orbital Diagram In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The orbital diagram for copper (cu) can be represented as follows: In the case of copper, the electron configuration is [ar]. The electron configuration of copper is [ar] 3d10 4s1. Copper has a unique electron configuration that can be represented using an orbital. Copper Electron Configuration Orbital Diagram.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Electron Configuration Orbital Diagram To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. Copper (cu) has an atomic number of 29,. The orbital diagram for copper (cu) can be represented as follows: Since 1s can only hold two electrons the next 2. Copper has a unique electron configuration that can be represented using an orbital. Copper Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Orbital Diagram For Copper Copper Electron Configuration Orbital Diagram An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. Copper has a unique electron configuration that can be represented using an orbital diagram. The orbital diagram. Copper Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Orbital Diagram For Copper Copper Electron Configuration Orbital Diagram Each box represents one orbital, and each arrow indicates one electron. Copper has a unique electron configuration that can be represented using an orbital diagram. Since 1s can only hold two electrons the next 2. For example, the orbital diagram of li can be shown as: 1s2 2s2 2p6 3s2 3p6 4s2 3d9. In writing the electron configuration for copper. Copper Electron Configuration Orbital Diagram.
From stewart-switch.com
Orbital Diagram For Copper Copper Electron Configuration Orbital Diagram The electron configuration of copper is [ar] 3d10 4s1. Copper has a unique electron configuration that can be represented using an orbital diagram. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. This is a way of showing the electron configuration of the atom. 1s2 2s2 2p6 3s2 3p6 4s2 3d9.. Copper Electron Configuration Orbital Diagram.
From valenceelectrons.com
Copper(Cu) electron configuration and orbital diagram Copper Electron Configuration Orbital Diagram Each box represents one orbital, and each arrow indicates one electron. In the case of copper, the electron configuration is [ar]. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. Copper (cu) has an atomic number of 29,. The orbital diagram for copper (cu) can be represented as follows: This means that copper has a completely filled 3d orbital with 10 electrons. Copper Electron Configuration Orbital Diagram.
From www.slideshare.net
Copper Copper Electron Configuration Orbital Diagram Since 1s can only hold two electrons the next 2. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. In the case of copper, the electron configuration is [ar]. Copper has a unique electron configuration that can be represented using an orbital diagram. In writing the electron configuration for copper the first two. Copper Electron Configuration Orbital Diagram.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Copper Electron Configuration Orbital Diagram In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Each box represents one orbital, and each arrow indicates one electron. The electron configuration of copper is [ar] 3d10 4s1. This means that copper has a completely filled 3d orbital with 10 electrons and one. For example, the orbital diagram. Copper Electron Configuration Orbital Diagram.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electron Configuration Orbital Diagram In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. This is a way of showing the electron configuration of the atom. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. An orbital diagram, like those shown above, is a visual. Copper Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Copper Orbital Diagram Copper Electron Configuration Orbital Diagram This is a way of showing the electron configuration of the atom. Each box represents one orbital, and each arrow indicates one electron. This means that copper has a completely filled 3d orbital with 10 electrons and one. To write the orbital diagram for the copper (cu) first we need to write the electron configuration for. In writing the electron. Copper Electron Configuration Orbital Diagram.
From mungfali.com
Orbital Diagram Of Copper Copper Electron Configuration Orbital Diagram In writing the electron configuration for copper the first two electrons will go in the 1s orbital. This means that copper has a completely filled 3d orbital with 10 electrons and one. The electron configuration of copper is [ar] 3d10 4s1. Copper (cu) has an atomic number of 29,. An orbital diagram, like those shown above, is a visual way. Copper Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Orbital Diagram For Copper Copper Electron Configuration Orbital Diagram Copper (cu) has an atomic number of 29,. The electron configuration of copper is [ar] 3d10 4s1. The electron configuration of copper refers to the arrangement of electrons in the copper atom’s orbitals. Copper has a unique electron configuration that can be represented using an orbital diagram. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in. Copper Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Orbital Diagram For Copper Copper Electron Configuration Orbital Diagram Since 1s can only hold two electrons the next 2. In the case of copper, the electron configuration is [ar]. This is a way of showing the electron configuration of the atom. This means that copper has a completely filled 3d orbital with 10 electrons and one. The electron configuration of copper refers to the arrangement of electrons in the. Copper Electron Configuration Orbital Diagram.
From manualpentathlon.z14.web.core.windows.net
Electron Configuration And Orbital Diagram Copper Electron Configuration Orbital Diagram This is a way of showing the electron configuration of the atom. In the case of copper, the electron configuration is [ar]. The orbital diagram for copper (cu) can be represented as follows: Copper (cu) has an atomic number of 29,. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper has. Copper Electron Configuration Orbital Diagram.
From manuallistcantabank.z21.web.core.windows.net
Electron Configuration Orbital Diagram Copper Electron Configuration Orbital Diagram Copper (cu) has an atomic number of 29,. Since 1s can only hold two electrons the next 2. Each box represents one orbital, and each arrow indicates one electron. In the case of copper, the electron configuration is [ar]. Copper has a unique electron configuration that can be represented using an orbital diagram. The electron configuration of copper is [ar]. Copper Electron Configuration Orbital Diagram.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Copper Electron Configuration Orbital Diagram In the case of copper, the electron configuration is [ar]. The orbital diagram for copper (cu) can be represented as follows: Copper has a unique electron configuration that can be represented using an orbital diagram. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. This means that copper has a completely filled 3d orbital with 10 electrons and one. An orbital diagram,. Copper Electron Configuration Orbital Diagram.
From valenceelectrons.com
Copper(Cu) electron configuration and orbital diagram Copper Electron Configuration Orbital Diagram For example, the orbital diagram of li can be shown as: The orbital diagram for copper (cu) can be represented as follows: Copper (cu) has an atomic number of 29,. Copper has a unique electron configuration that can be represented using an orbital diagram. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows. Copper Electron Configuration Orbital Diagram.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Copper (Cu) YouTube Copper Electron Configuration Orbital Diagram This is a way of showing the electron configuration of the atom. Since 1s can only hold two electrons the next 2. Copper (cu) has an atomic number of 29,. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the. In writing the electron configuration. Copper Electron Configuration Orbital Diagram.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Electron Configuration Orbital Diagram The electron configuration of copper is [ar] 3d10 4s1. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. For example, the orbital diagram of li can be shown as: Since 1s can only hold two electrons the next 2. Each box represents one orbital, and each arrow indicates one electron. The orbital. Copper Electron Configuration Orbital Diagram.
From ar.inspiredpencil.com
Copper Orbital Diagram Copper Electron Configuration Orbital Diagram Copper has a unique electron configuration that can be represented using an orbital diagram. Each box represents one orbital, and each arrow indicates one electron. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the. This means that copper has a completely filled 3d orbital. Copper Electron Configuration Orbital Diagram.