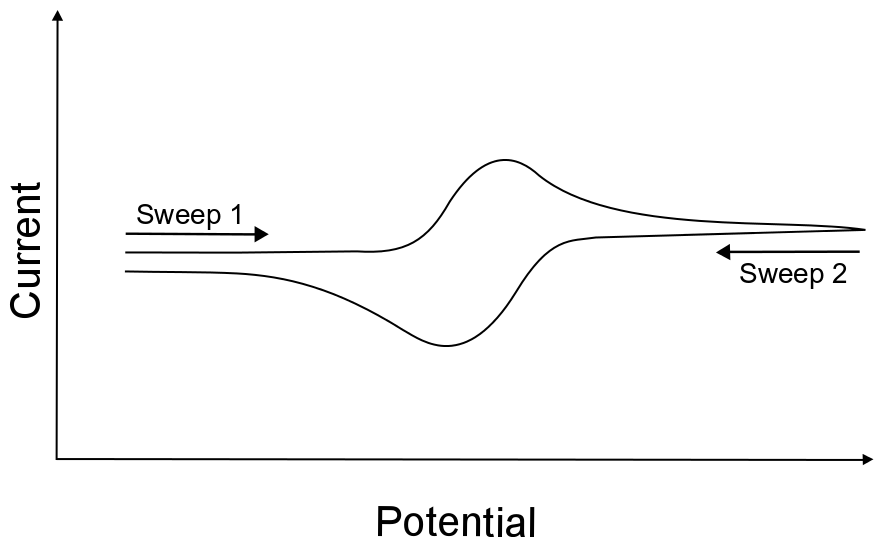
Electron-Transfer Kinetics From Cyclic Voltammetry . Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. When there is a low.
from sop4cv.com
Quantitative description of electrochemical reversibility. When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte.
What Is Cyclic Voltammetry?
Electron-Transfer Kinetics From Cyclic Voltammetry A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. When there is a low.
From www.researchgate.net
Cyclic voltammetry curves of electrochemical capacitors with different Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Cyclic voltammetry model for obtaining electron transfer for a Electron-Transfer Kinetics From Cyclic Voltammetry A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. When there is a low. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From chemistnotes.com
Cyclic Voltammetry Basic principle Chemistry Notes Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
a Cyclic voltammetry curves of TiO2, CQDs, and CQDs20/TiO2, b the Electron-Transfer Kinetics From Cyclic Voltammetry A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Quantitative description of electrochemical reversibility. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Plot of electron transfer versus cyclic voltammetric scan rate Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Cyclic voltammetry Cyclic voltammogram for TiO2 and TiO2(RuP²⁺)5,1 in Electron-Transfer Kinetics From Cyclic Voltammetry A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Quantitative description of electrochemical reversibility. When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.semanticscholar.org
Figure 4 from FastScan Cyclic Voltammetry Allows Determination of Electron-Transfer Kinetics From Cyclic Voltammetry Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From pubs.acs.org
Cyclic Voltammetry of Palladium(II) Complexes with Tridentate Arsine Electron-Transfer Kinetics From Cyclic Voltammetry Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.numerade.com
SOLVED Transient cyclic voltammetry is a very powerful technique to Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Quantitative description of electrochemical reversibility. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.slideserve.com
PPT Thin Film Cyclic Voltammetry PowerPoint Presentation, free Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.semanticscholar.org
Figure 1 from NonNernstian TwoElectron Transfer Reactions for Electron-Transfer Kinetics From Cyclic Voltammetry Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.youtube.com
Electron transfer followed by chemical reaction cyclic voltammetry part Electron-Transfer Kinetics From Cyclic Voltammetry A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Quantitative description of electrochemical reversibility. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
3. A comparison of cyclic voltammetric waveshapes for reversible Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.semanticscholar.org
Figure 1 from FastScan Cyclic Voltammetry Allows Determination of Electron-Transfer Kinetics From Cyclic Voltammetry A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Cyclic voltammetry of NPGL15 (a), NPGL30 (b), NPGL60 (c), and GCE (d Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Quantitative description of electrochemical reversibility. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Cyclic voltammetry model for obtaining electron transfer for a Electron-Transfer Kinetics From Cyclic Voltammetry A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. When there is a low. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Cyclic voltammetry model for obtaining electron transfer for a Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
6 Cyclic voltammetry of a simple electron transfer reaction, where a Electron-Transfer Kinetics From Cyclic Voltammetry A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Quantitative description of electrochemical reversibility. When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From materean.com
Cyclic voltammetry Paul Wu's Blog Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From slidetodoc.com
Lecture 6 a Cyclic Voltammetry Introduction I Electrochemical Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. Quantitative description of electrochemical reversibility. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electron-Transfer Kinetics From Cyclic Voltammetry.
From sop4cv.com
What Is Cyclic Voltammetry? Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.semanticscholar.org
Figure 1 from FastScan Cyclic Voltammetry Allows Determination of Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Quantitative description of electrochemical reversibility. When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.semanticscholar.org
[PDF] Transient Voltammetry with Ultramicroelectrodes Reveals the Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. Electron-Transfer Kinetics From Cyclic Voltammetry.
From slidetodoc.com
Lecture 6 a Cyclic Voltammetry Introduction I Electrochemical Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Scan rate studies (a) cyclic voltammetry at different scan rates from Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Quantitative description of electrochemical reversibility. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
A cyclic voltammogram illustrating the process of electron transfer Electron-Transfer Kinetics From Cyclic Voltammetry Quantitative description of electrochemical reversibility. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From slideplayer.com
Electrochemistry MAE ppt download Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Plot of electron transfer versus cyclic voltammetric scan rate Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Quantitative description of electrochemical reversibility. Electron-Transfer Kinetics From Cyclic Voltammetry.
From achs-prod.acs.org
FastScan Cyclic Voltammetry Allows Determination of ElectronTransfer Electron-Transfer Kinetics From Cyclic Voltammetry Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. When there is a low. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.semanticscholar.org
Figure 1 from FastScan Cyclic Voltammetry Allows Determination of Electron-Transfer Kinetics From Cyclic Voltammetry Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Cyclic voltammetry model for obtaining electron transfer for a Electron-Transfer Kinetics From Cyclic Voltammetry Quantitative description of electrochemical reversibility. When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Cyclic voltammetry of BAD and ferrocene (inset) dissolved in Electron-Transfer Kinetics From Cyclic Voltammetry Quantitative description of electrochemical reversibility. When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
Cyclic voltammetry of (a) TiO2 nanotubes and (b) NTiO2 nanotubes for 5 Electron-Transfer Kinetics From Cyclic Voltammetry Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. A commonly applied electroanalytical tool is cyclic voltammetry (cv). When there is a low. Quantitative description of electrochemical reversibility. Electron-Transfer Kinetics From Cyclic Voltammetry.
From www.researchgate.net
(a) Cyclic voltammetry of 5 mmol L −1 [Fe(CN) 6 ] 4−/3− on glassy Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Quantitative description of electrochemical reversibility. Electron-Transfer Kinetics From Cyclic Voltammetry.
From lysihuset.blogspot.com
Cyclic voltammetry Electron-Transfer Kinetics From Cyclic Voltammetry When there is a low. Quantitative description of electrochemical reversibility. A commonly applied electroanalytical tool is cyclic voltammetry (cv). Electrochemical reversibility refers to the electron transfer kinetics between the electrode and the analyte. Electron-Transfer Kinetics From Cyclic Voltammetry.