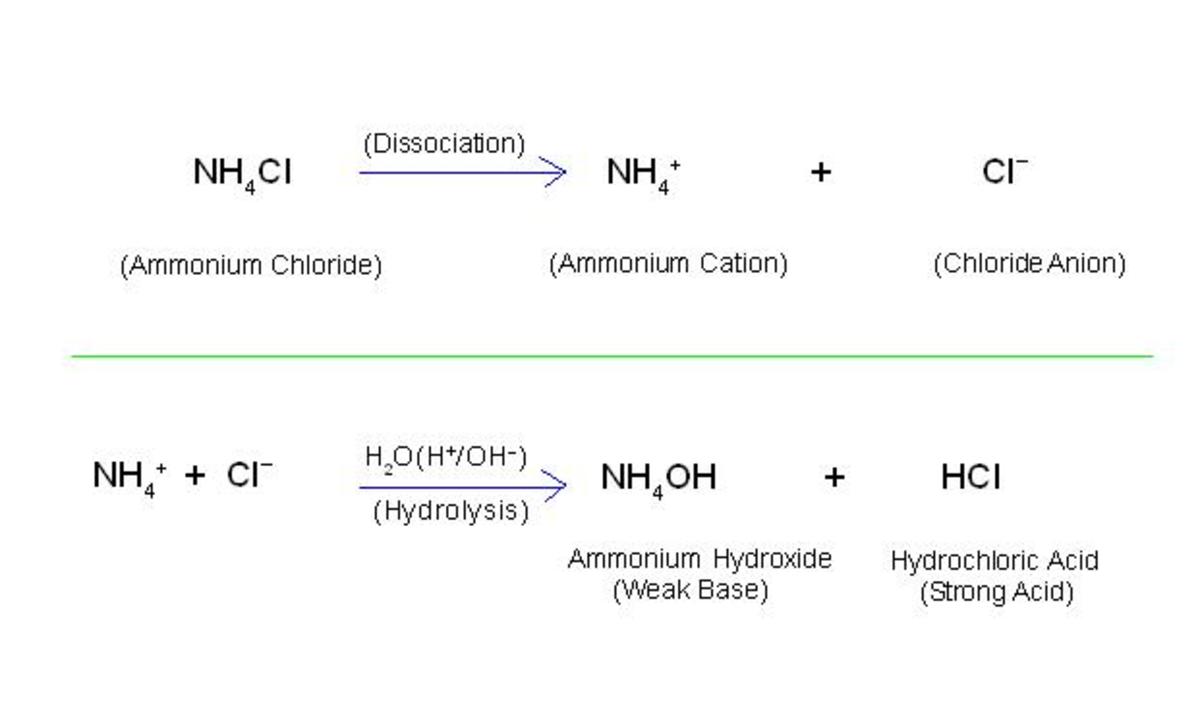
Hydrolysis Of Iron(Iii) Chloride Equation . in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. Iron is the lewis acid in this case (electron pair acceptor) and water is. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. The chemical equation for this reaction is provided below. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. Cationic hydrolysis is possible because. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. 2fe + 3cl 2 →. iron (iii) chloride dissolves in water via acid base chemistry.
from discover.hubpages.com
iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. Cationic hydrolysis is possible because. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. The chemical equation for this reaction is provided below. 2fe + 3cl 2 →. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. Iron is the lewis acid in this case (electron pair acceptor) and water is.
The Difference in the Chemistry of Hydrolysis & Hydration. HubPages
Hydrolysis Of Iron(Iii) Chloride Equation anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. 2fe + 3cl 2 →. The chemical equation for this reaction is provided below. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. Cationic hydrolysis is possible because. iron (iii) chloride dissolves in water via acid base chemistry. Iron is the lewis acid in this case (electron pair acceptor) and water is.
From courses.lumenlearning.com
Classifying Chemical Reactions Chemistry Hydrolysis Of Iron(Iii) Chloride Equation iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. The chemical equation for this reaction is provided below. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. when dissolved in water, it. Hydrolysis Of Iron(Iii) Chloride Equation.
From app.pandai.org
Chemical Formulae Hydrolysis Of Iron(Iii) Chloride Equation iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron (iii) chloride dissolves in water. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.youtube.com
Iron(III) chloride YouTube Hydrolysis Of Iron(Iii) Chloride Equation Cationic hydrolysis is possible because. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. The chemical equation for this reaction is provided below. iron(iii) chloride indeed hydrolyses in water,. Hydrolysis Of Iron(Iii) Chloride Equation.
From ar.inspiredpencil.com
Iron Chloride Hydrolysis Of Iron(Iii) Chloride Equation anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. The chemical equation for this reaction is provided below. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.numerade.com
SOLVED Iron metal reacts with chlorine gas according to the following Hydrolysis Of Iron(Iii) Chloride Equation iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron (iii) chloride dissolves in water via acid base chemistry. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Hydrolysis Of Iron(Iii) Chloride Equation when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. Cationic hydrolysis is possible because. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. Iron is the lewis acid in this case (electron pair acceptor) and water is. iron(iii) chloride indeed hydrolyses in water,. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation, free Hydrolysis Of Iron(Iii) Chloride Equation iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. Iron is the lewis acid in this case (electron pair acceptor) and water is. Cationic hydrolysis is possible because. iron(iii) chloride,. Hydrolysis Of Iron(Iii) Chloride Equation.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Hydrolysis Of Iron(Iii) Chloride Equation The chemical equation for this reaction is provided below. Iron is the lewis acid in this case (electron pair acceptor) and water is. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. 2fe + 3cl 2 →. iron (iii) chloride dissolves in water via acid base chemistry. the hydrolysis of iron(iii) chloride is. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.youtube.com
Equation for FeCl3 + H2O Iron (III) chloride + Water YouTube Hydrolysis Of Iron(Iii) Chloride Equation the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. The chemical equation for this reaction is provided below. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.numerade.com
SOLVEDWhat would be formed by the electrolysis of an acidic iron(III Hydrolysis Of Iron(Iii) Chloride Equation in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. iron (iii) chloride dissolves in water via acid base chemistry. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. iron(iii) chloride, also known as ferric chloride, is a compound. Hydrolysis Of Iron(Iii) Chloride Equation.
From melscience.com
The hydrolysis of iron(III) chloride MEL Chemistry Hydrolysis Of Iron(Iii) Chloride Equation Iron is the lewis acid in this case (electron pair acceptor) and water is. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. iron(iii) chloride indeed hydrolyses. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.toppr.com
Iron III Chloride Formula Structure, Preparations and Properties Hydrolysis Of Iron(Iii) Chloride Equation iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. Cationic hydrolysis is possible because. iron (iii) chloride dissolves in water via acid base chemistry. when dissolved in water, it undergoes hydrolysis,. Hydrolysis Of Iron(Iii) Chloride Equation.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Hydrolysis Of Iron(Iii) Chloride Equation anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. Cationic hydrolysis is possible because. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. iron (iii) chloride dissolves in water via acid base chemistry. the hydrolysis of iron(iii) chloride is the cationic reaction of. Hydrolysis Of Iron(Iii) Chloride Equation.
From discover.hubpages.com
The Difference in the Chemistry of Hydrolysis & Hydration. HubPages Hydrolysis Of Iron(Iii) Chloride Equation when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. 2fe + 3cl 2 →. Cationic hydrolysis is possible because. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.bartleby.com
Answered The Iron metal reacts with chlorine gas… bartleby Hydrolysis Of Iron(Iii) Chloride Equation Cationic hydrolysis is possible because. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. 2fe + 3cl 2 →. iron (iii) chloride dissolves in water via acid base chemistry. The chemical equation for. Hydrolysis Of Iron(Iii) Chloride Equation.
From mungfali.com
Hydrolysis Reaction Diagram Hydrolysis Of Iron(Iii) Chloride Equation in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. Cationic hydrolysis is possible because. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron (iii) chloride dissolves in water via acid base chemistry. 2fe + 3cl 2 →. The chemical. Hydrolysis Of Iron(Iii) Chloride Equation.
From mungfali.com
Hydrolysis Reaction Equation Hydrolysis Of Iron(Iii) Chloride Equation Iron is the lewis acid in this case (electron pair acceptor) and water is. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. 2fe + 3cl 2 →. in this video we will. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.shutterstock.com
Iron Iii Chloride Handwritten Chemical Formula Stock Illustration Hydrolysis Of Iron(Iii) Chloride Equation 2fe + 3cl 2 →. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. Cationic hydrolysis is possible because. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium.. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.slideserve.com
PPT COORDINATION CHEMISTRY in Solution) PowerPoint Hydrolysis Of Iron(Iii) Chloride Equation The chemical equation for this reaction is provided below. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. Cationic hydrolysis is possible because. the hydrolysis of iron(iii). Hydrolysis Of Iron(Iii) Chloride Equation.
From fphoto.photoshelter.com
science chemistry iron chloride hydrolysis Fundamental Photographs Hydrolysis Of Iron(Iii) Chloride Equation Iron is the lewis acid in this case (electron pair acceptor) and water is. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. 2fe + 3cl 2 →. in this video we will. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.youtube.com
How to write the formula for iron (III) chloride YouTube Hydrolysis Of Iron(Iii) Chloride Equation 2fe + 3cl 2 →. Cationic hydrolysis is possible because. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron(iii) chloride, also known as ferric chloride, is a compound of iron. Hydrolysis Of Iron(Iii) Chloride Equation.
From peacecommission.kdsg.gov.ng
Iron(III) Chloride Hydrolysis Of Iron(Iii) Chloride Equation in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. Cationic hydrolysis is possible because. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. 2fe + 3cl 2 →. iron (iii) chloride dissolves in water via acid base chemistry. the hydrolysis. Hydrolysis Of Iron(Iii) Chloride Equation.
From brainly.in
aqueous iron chloride and sodium carbonate solution yields aqueous Hydrolysis Of Iron(Iii) Chloride Equation iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. 2fe + 3cl 2 →. Iron is the lewis acid in this case (electron pair acceptor) and water is. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. when dissolved in water,. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.coursehero.com
[Solved] A chemist prepares a solution of iron (III) chloride (FeCl Hydrolysis Of Iron(Iii) Chloride Equation when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. Iron is the lewis acid in this case (electron pair acceptor) and water is. 2fe + 3cl 2 →. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. Cationic hydrolysis is possible because. iron(iii) chloride. Hydrolysis Of Iron(Iii) Chloride Equation.
From exombjkja.blob.core.windows.net
Iron 3 Chloride Ligands at Linda Chase blog Hydrolysis Of Iron(Iii) Chloride Equation when dissolved in water, it undergoes hydrolysis, resulting in the formation of hydrochloric acid and iron (iii) hydroxide. Iron is the lewis acid in this case (electron pair acceptor) and water is. Cationic hydrolysis is possible because. The chemical equation for this reaction is provided below. iron(iii) chloride, also known as ferric chloride, is a compound of iron. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.youtube.com
Write the formula for iron(III) chloride YouTube Hydrolysis Of Iron(Iii) Chloride Equation anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. 2fe + 3cl 2 →. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. in this video we will describe the equation fecl3. Hydrolysis Of Iron(Iii) Chloride Equation.
From molekula.com
Purchase Iron(III) chloride anhydrous [7705080] online • Catalog Hydrolysis Of Iron(Iii) Chloride Equation Iron is the lewis acid in this case (electron pair acceptor) and water is. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. iron (iii) chloride dissolves in water via acid base chemistry. The chemical equation for this reaction is provided below. when dissolved in water, it. Hydrolysis Of Iron(Iii) Chloride Equation.
From testbook.com
Iron (III) Chloride Formula Know Structure, Preparation,& Uses Hydrolysis Of Iron(Iii) Chloride Equation 2fe + 3cl 2 →. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. The chemical equation for this reaction is provided below. Cationic hydrolysis is possible because. Iron is the lewis acid in this case (electron pair acceptor) and water is. iron(iii) chloride, also known. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.bartleby.com
The reaction of iron metal and chlorine gas to give iron(III) chloride Hydrolysis Of Iron(Iii) Chloride Equation iron (iii) chloride dissolves in water via acid base chemistry. Iron is the lewis acid in this case (electron pair acceptor) and water is. Cationic hydrolysis is possible because. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. iron(iii) chloride, also known as ferric chloride,. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.numerade.com
Challenge Solid iron reacts with chlorine gas to form solid iron(llI Hydrolysis Of Iron(Iii) Chloride Equation Cationic hydrolysis is possible because. Iron is the lewis acid in this case (electron pair acceptor) and water is. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron(iii) chloride, also known as ferric chloride, is a compound of iron and chlorine with the formula fecl 3. when dissolved in water, it. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.youtube.com
How to Balance FeCl3 + Na2S = Fe2S3 + NaCl (Iron (III) chloride Hydrolysis Of Iron(Iii) Chloride Equation anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. Iron is the lewis acid in this case (electron pair acceptor) and water is. the hydrolysis of iron(iii) chloride is the cationic reaction of the salt with water. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. . Hydrolysis Of Iron(Iii) Chloride Equation.
From www.chegg.com
Chemistry Archive June 29, 2017 Hydrolysis Of Iron(Iii) Chloride Equation anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. iron (iii) chloride dissolves in water via acid base chemistry. Cationic hydrolysis is possible because. iron(iii) chloride, also known as ferric chloride, is a compound of iron and. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.researchgate.net
What is the chemical difference between these iron III chloride Hydrolysis Of Iron(Iii) Chloride Equation anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. The chemical equation for this reaction is provided below. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. 2fe + 3cl 2 →. iron (iii) chloride dissolves in water via acid base. Hydrolysis Of Iron(Iii) Chloride Equation.
From dxotceedv.blob.core.windows.net
Hydrochloric Acid To Produce Magnesium Chloride And Hydrogen Gas at Hydrolysis Of Iron(Iii) Chloride Equation iron (iii) chloride dissolves in water via acid base chemistry. in this video we will describe the equation fecl3 + h2o and write what happens when fecl3 (iron (iii) chloride) is. Iron is the lewis acid in this case (electron pair acceptor) and water is. Cationic hydrolysis is possible because. The chemical equation for this reaction is provided. Hydrolysis Of Iron(Iii) Chloride Equation.
From www.youtube.com
How to Write the Formula for Iron (III) chloride YouTube Hydrolysis Of Iron(Iii) Chloride Equation Iron is the lewis acid in this case (electron pair acceptor) and water is. iron(iii) chloride indeed hydrolyses in water, or to be precise, $\ce{fe^3+}$ does assuring acidic medium. 2fe + 3cl 2 →. anhydrous iron (iii) chloride can be prepared by reacting metallic iron with dichloride. iron(iii) chloride, also known as ferric chloride, is a compound. Hydrolysis Of Iron(Iii) Chloride Equation.